BEDFORD, Mass., Oct. 04, 2016 (GLOBE NEWSWIRE) — ConforMIS, Inc. (NASDAQ:CFMS), a medical technology company that develops, manufactures and sells joint replacement implants that are customized to fit each patient’s unique anatomy, today announced a significant milestone. ConforMIS has sold more than 50,000 implants, each individually sized and shaped to fit each patient’s unique anatomy. Unlike manufacturers of traditional “off-the-shelf” knee replacement implants that offer products with a limited range of sizes and geometries, ConforMIS offers a broad line of customized knee implants designed to restore the natural shape of a patient’s knee. To commemorate this milestone, a group of male and female patients, ages 40-75, will be gathering in Boston to kick-off the ConforMIS Patient Ambassador Program.
“We are thrilled to be commemorating this important company milestone together with our deeply committed team of ConforMIS employees dedicated to delivering innovative, high quality technology. We are in the business of helping surgeons and healthcare professionals treat their knee replacement patients by offering them a broad line of customized knee implants for individualized orthopedic care. Understanding that patient-to-patient communication is one of the most meaningful experiences for potential patients seeking joint replacement options, we are excited to introduce a new program designed to facilitate and enable patient connectivity,” said Philipp Lang, MD, MBA, Chief Executive Officer and President of ConforMIS. “With over 50,000 implants sold, we feel the timing is right to launch this initiative. Our patients are unique, each and every one of them, and our Patient Ambassador Program will allow the everyday ConforMIS patient to share his or her story with others fighting a similar battle.”
This week ConforMIS is holding its first Patient Ambassador Summit, a special gathering of patients from across the United States treated with ConforMIS customized knee implants in one or both knees. This group includes some of the earliest patients to be treated with a ConforMIS knee implant alongside more recent patients. The Patient Ambassador group is comprised of patients that have either a partial or total ConforMIS customized knee replacement ranging from the iUni, iTotal CR and iTotal PS, and each will share their personal story. This week, these patients will have the opportunity to experience firsthand how ConforMIS solutions are individually designed and manufactured using its proprietary software and 3D printing technology.
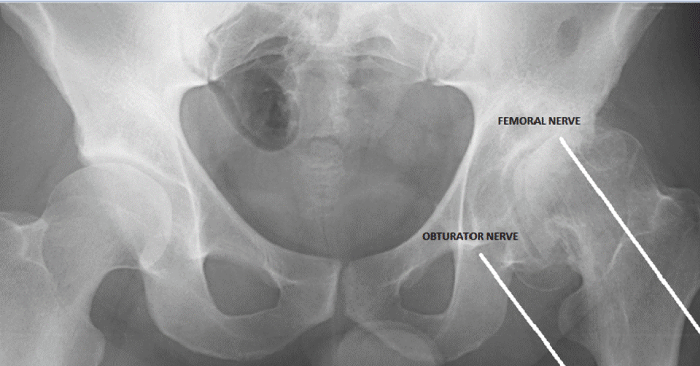
Each ConforMIS partial and total knee implant, including iTotal® CR, iTotal® PS, iDuo® and iUni®, is developed using proprietary algorithms and computer software to map the articular surfaces of the knee joint, define the areas of disease and convert the imaging data into a three-dimensional model of the knee. ConforMIS engineers then use computer-aided design, or CAD, software to design the customized implant and single-use, sterile surgical instrumentation that will precisely match the three dimensional model of the patient’s knee.
The focus on innovation in customized implants continues today at ConforMIS. In March 2016 the company launched iTotal PS, which nearly triples the company’s addressable market. Like iTotal CR, iTotal PS implants are customized for each patient to avoid compromises on implant fit, rotation and alignment, which can cause residual pain and functional limitations after surgery.
About ConforMIS, Inc.
ConforMIS is a medical technology company that uses its proprietary iFit Image-to-Implant technology platform to develop, manufacture and sell joint replacement implants that are individually sized and shaped, or customized, to fit each patient’s unique anatomy. ConforMIS offers a broad line of customized knee implants and pre-sterilized, single-use instruments delivered in a single package to the hospital. In recent clinical studies, ConforMIS iTotal CR demonstrated superior clinical outcomes, including better function and greater patient satisfaction, compared to traditional, off-the-shelf implants. ConforMIS owns or exclusively in-licenses approximately 500 issued patents and pending patent applications that cover customized implants and patient-specific instrumentation for all major joints.
For more information, visit www.conformis.com. To receive future releases in e-mail alerts, sign up athttp://ir.conformis.com/.
Cautionary Statement Regarding Forward-Looking Statements
Any statements in this press release about future expectations, plans and prospects for ConforMIS, including statements about the Patient Ambassador Program, the potential clinical, economic or other impacts and advantages of using customized implants, as well as other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would” and similar expressions, constitute forward-looking statements within the meaning of the safe harbor provisions of The Private Securities Litigation Reform Act of 1995. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make as a result of a variety of risks and uncertainties, including risks related to results seen in ongoing and future clinical and economic studies of our products, risks related to our estimates regarding the potential market opportunity for our current and future products, our expectations regarding our sales and other results of operations, the impact of patient communication programs, and the other risks and uncertainties described in the “Risk Factors” sections of our public filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent ConforMIS’s views as of the date hereof. ConforMIS anticipates that subsequent events and developments may cause ConforMIS’s views to change. However, while ConforMIS may elect to update these forward-looking statements at some point in the future, ConforMIS specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing ConforMIS’s views as of any date subsequent to the date hereof.
CONTACT:
Investor contact:
Oksana Bradley
ir@conformis.com
(781) 374-5598
Media contacts:
Bill Berry
Berry & Company Public Relations
Bberry@berrypr.com
(212) 253-8881
Lynn Granito
Berry & Company Public Relations
Lgranito@berrypr.com
(212) 253-8881