June 05, 2017
DURHAM, N.C.–(BUSINESS WIRE)–Low-intensity pulsed ultrasound (LIPUS) treatment can be an effective alternative to surgery for nonunions, which are fractures that fail to heal. This according to a systematic review and meta-analysis of 13 published studies that describe nonunions treated with LIPUS. The findings were published in the May 15, 2017 issue of Injury available at http://www.injuryjournal.com/article/S0020-1383(17)30341-8/fulltext.
Nonunions occur in approximately 5% of patient fracture cases each year. Nonunions have little expectation of spontaneous healing and can be corrected with revision surgery, however such procedures typically pose technical challenges and an increased potential for complications. When surgery is optional and patients want to avoid its associated risks, choosing LIPUS has demonstrated a positive healing effect more than 80% of the time, which is comparable to the success rate for surgical treatment.
“This meta-analysis revealed that LIPUS is a viable alternative to surgery for established nonunions,” said Dr. R. Grant Steen, Manager of Medical Affairs, Bioventus. “The heal rate across all primary studies included was 82% and increased to 84% for nonunions that were eight months or older.”
The review and meta-analysis was funded by Bioventus and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Studies were considered eligible for review if LIPUS was used as an alternative to surgery for non-healing fractures and if the treatment was applied at least three months after the last surgical procedure. In addition, at least one outcome of interest (heal/fail) was required and a clear definition of delayed or nonunion was included.
Authors of this study include Ross Leighton, MD, Dalhousie University; J. Tracy Watson, MD, Saint Louis University School of Medicine; Peter Giannoudis, MD, University of Leeds; Costas Papakostidis, MD, Chapel Allerton Hospital; Andrew Harrison, PhD, Bioventus and R. Grant Steen, PhD, Bioventus.
About Bioventus
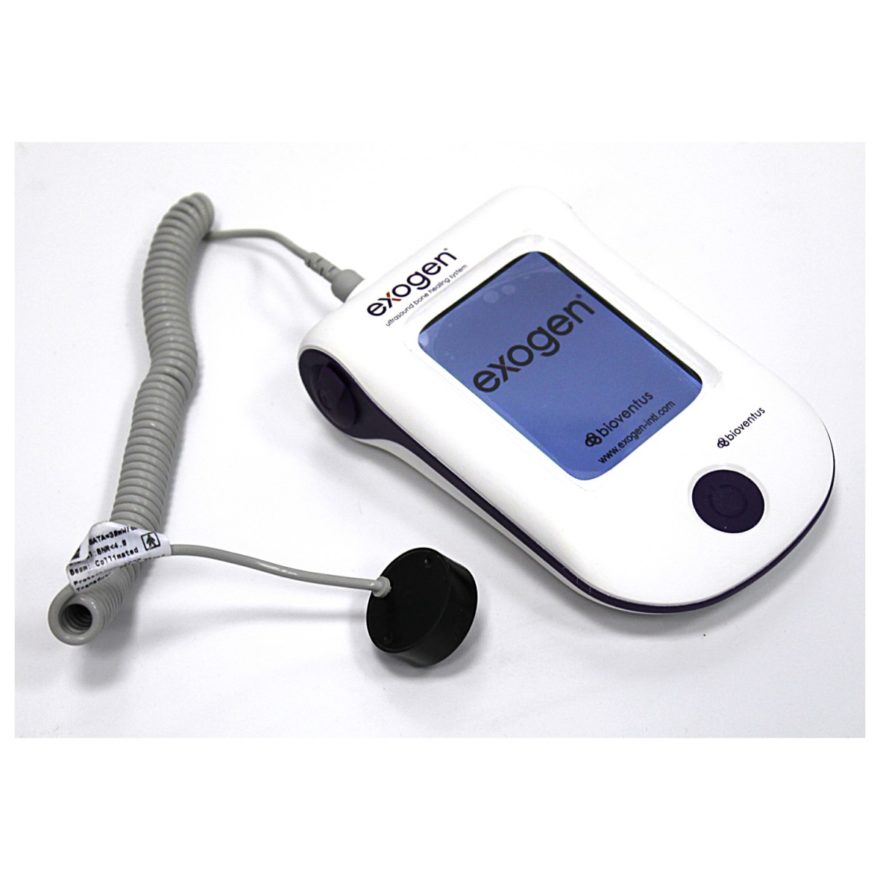
Bioventus is an orthobiologics company that delivers clinically proven, cost-effective products that help people heal quickly and safely. Its mission is to make a difference by helping patients resume and enjoy active lives. The company has two product portfolios for orthobiologics, Bioventus Active Healing Therapies and Bioventus Surgical that make it a global leader in active orthopaedic healing. Its EXOGEN® Ultrasound Bone Healing System uses safe, effective low intensity pulsed ultrasound (LIPUS) to stimulate the body’s natural healing process. EXOGEN has been used to treat more than 1 million patients worldwide and numerous regulatory agencies including the FDA, Health Canada, BSi, TGA, Medsafe, UAE Ministry of Health and SFDA have granted their approval of the product. Today it is the leading bone healing system in the market with complaints for lack of efficacy averaging less than 1%.
Built on a commitment to high quality standards, evidence-based medicine and strong ethical behavior, Bioventus is a trusted partner for physicians worldwide. For more information, visit www.BioventusGlobal.com and follow the company on Twitter @Bioventusglobal.
Bioventus, the Bioventus logo and EXOGEN are registered trademarks of Bioventus LLC.
Contacts
Bioventus LLC
Thomas Hill, 919-474-6715
thomas.hill@bioventusglobal.com