September 25, 2018
 LOS ANGELES–(BUSINESS WIRE)–RTI Surgical, Inc. (Nasdaq:RTIX), a global surgical implant company, announced its TETRAfuse® 3D Technology won a 2018 Spine Technology Award from Orthopedics This Week, a widely read publication in the orthopedics industry. RTI will accept the award at the North American Spine Society’s (NASS) 33rd Annual Meeting taking place September 26-29, 2018 in Los Angeles and will be featured in upcoming issues of Orthopedics This Week and Orthopedics This Month Spine.
LOS ANGELES–(BUSINESS WIRE)–RTI Surgical, Inc. (Nasdaq:RTIX), a global surgical implant company, announced its TETRAfuse® 3D Technology won a 2018 Spine Technology Award from Orthopedics This Week, a widely read publication in the orthopedics industry. RTI will accept the award at the North American Spine Society’s (NASS) 33rd Annual Meeting taking place September 26-29, 2018 in Los Angeles and will be featured in upcoming issues of Orthopedics This Week and Orthopedics This Month Spine.
“RTI is honored to receive this important industry recognition, which is a testament to the ingenuity and dedication of our employees,” said Camille Farhat, President and CEO, RTI Surgical. “TETRAfuse 3D Technology is one of many examples of RTI’s commitment to the development and ongoing clinical research of innovative spine-focused solutions that meet the demands of surgeons and improve patient outcomes around the world.”
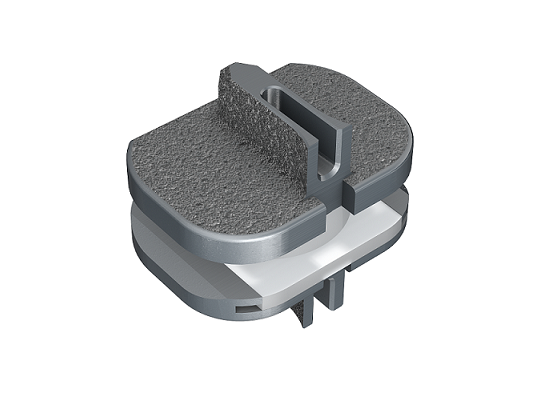
TETRAfuse 3D Technology is an interbody fusion (IBF) device material, manufactured under an exclusive license with Oxford Performance Materials, designed to help drive optimal outcomes for patients undergoing spinal fusion procedures. It is the first 3D printed polymer-based IBF device material to incorporate a nano-roughisurface that has demonstrated, in a pre-clinical study, more notable trabecular bone ingrowth compared to PEEK and titanium-coated PEEK.ii
 TETRAfuse 3D Technology is featured in RTI’s growing Fortilink® series of devices, including the Fortilink-C, -TS and -L IBF Systems. The devices are intended for use in interbody fusion procedures in patients with degenerative disc disease (DDD), an age-related condition when one or more discs between the vertebrae of the spinal column deteriorate or break down, which can lead to pain.iii
TETRAfuse 3D Technology is featured in RTI’s growing Fortilink® series of devices, including the Fortilink-C, -TS and -L IBF Systems. The devices are intended for use in interbody fusion procedures in patients with degenerative disc disease (DDD), an age-related condition when one or more discs between the vertebrae of the spinal column deteriorate or break down, which can lead to pain.iii
Those attending NASS can visit Booth #1523 to learn more about the Fortilink series of devices featuring TETRAfuse 3D Technology, as well as RTI’s full line of high-quality hardware, interbody and orthobiologic spine offerings. For more information about the Fortilink IBF series of devices and TETRAfuse 3D Technology, visit www.tetrafuse3D.com.
About Orthopedics This Week
Orthopedics This Week delivers breaking news, analysis and commentary to professionals working in the orthopedics industry. Published 40 times a year, Orthopedics This Week is a four-time winner of the MORE awards for journalistic excellence. Each year, Orthopedics This Week grants the Spine Technology Awards to inventors, engineering teams, surgeons and their companies who have created the most innovative, enduring and practical products in the treatment or care of the spine. Submissions are judged by a panel of leading surgeons with extensive clinical and research experience in spinal care.
About RTI Surgical, Inc.
RTI Surgical is a leading global surgical implant company providing surgeons with safe biologic, metal and synthetic implants. Committed to delivering a higher standard, RTI’s implants are used in sports medicine, general surgery, spine, orthopedic and trauma procedures and are distributed in nearly 50 countries. RTI has four manufacturing facilities throughout the U.S. and Europe. RTI is accredited in the U.S. by the American Association of Tissue Banks and is a member of AdvaMed. For more information, please visit www.rtix.com. Connect with us on LinkedIn and Twitter.
Forward-Looking Statements
This communication contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on management’s current expectations, estimates and projections about our industry, our management’s beliefs and certain assumptions made by our management. Words such as “anticipates,” “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” variations of such words and similar expressions are intended to identify such forward-looking statements. In addition, except for historical information, any statements made in this communication about anticipated financial results, growth rates, new product introductions, future operational improvements, gaining market share and results or regulatory actions or approvals or changes to agreements with distributors also are forward-looking statements. These statements are not guarantees of future performance and are subject to risks and uncertainties, including the risks described in public filings with the U.S. Securities and Exchange Commission (SEC). Our actual results may differ materially from the anticipated results reflected in these forward-looking statements. Copies of the company’s SEC filings may be obtained by contacting the company or the SEC or by visiting RTI’s website at www.rtix.com or the SEC’s website at www.sec.gov.
i Data on file at RTI Surgical, Inc.
ii Data on file at RTI Surgical, Inc. Performance data from animal studies may not be representative of performance in humans.
iii Donally C, Dulebohn S; Lumbar Degenerative Disk Disease. StatPearls. 2017 Oct 13. Available at https://www.ncbi.nlm.nih.gov/books/NBK448134/
Contacts
RTI Surgical, Inc.
Media Contacts:
Annie Claggett, +1 312-995-2856
aclaggett@rtix.com
or
Molly Poarch, +1 224-287-2661
mpoarch@rtix.com
or
Investor Contact:
Nathan Elwell, +1 847-530-0249