Smith & Nephew (LSE:SN, NYSE:SNN), the global medical technology business, today announces the publication of new clinical evidence highlighting improved patient outcomes following orthopaedic surgery.1
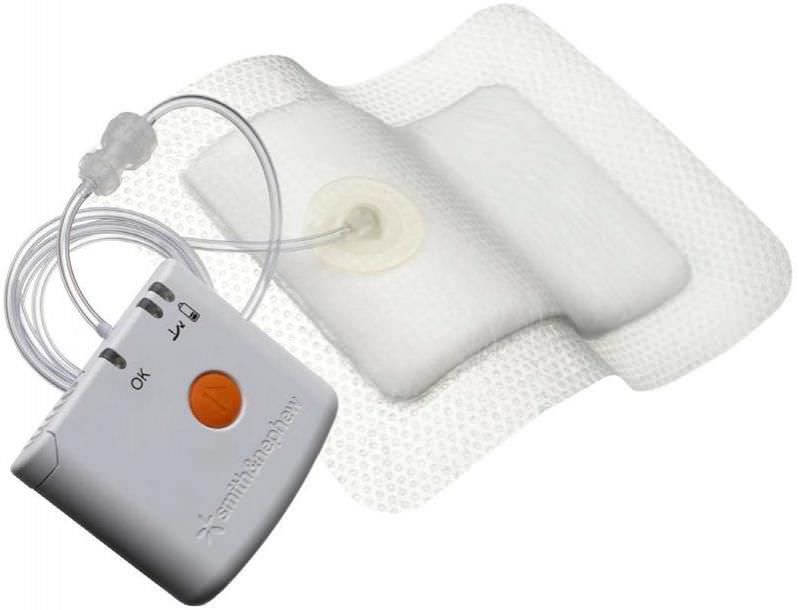
A Randomised Controlled Trial (RCT) of 220 patients undergoing primary hip or knee replacement compared the use of PICO, the novel Single use Negative Pressure Wound Therapy (NPWT) system, with standard dressings on closed surgical incisions. The research took place over a 12 month period at The Robert Jones and Agnes Hunt Orthopaedic Hospital NHS Foundation Trust (RJAH), Shropshire, UK. Results show significant reduction in; wound exudate distribution within the dressing, the number of dressing changes and extreme length of stay in hospital amongst those patients where PICO was applied.1
Whilst Total Hip Arthroplasty (THA) and Total Knee Arthroplasty (TKA) are commonplace procedures, reported figures of complications vary. Surgical site infection rate is suggested to be between 1-3% in the general orthopaedic population2, however UK Patient Reported Outcome Measures note that post-operative wound problems are recorded by approximately 10-13% of patients.3
Mr Sudheer Karlakki, lead RCT author and Consultant Orthopaedic Surgeon at RJAH, explains, “By introducing PICO as a prophylactic measure we have been able to demonstrate predictable wound healing following total hip and knee replacement procedures. Better wound management offers significant value to our hospital both in terms of reducing associated healthcare costs and by improving each patient’s outcome and experience.”
The impact of post-operative complications and prolonged wound exudate is serious and can lead to delayed discharge, increasing the care and cost burden to the hospital.4 The cost of any additional stay in a UK hospital is estimated at £275.00 per day, per hospital bed.1 Findings indicate that the use of PICO resulted in patients having a significantly smaller probability of experiencing excessive Length of Stay (LOS). The study showed a smaller range and spread of LOS in those patients with PICO compared to the standard dressing (1-10 days compared to 2-61 days).1
The use of PICO resulted in a four-fold reduction in the number of patients experiencing Grade 4 distribution of wound exudate within the dressing when compared with standard wound dressing in those undergoing joint replacement.1 Prolonged wound drainage and high exudate levels increase the risk of wound complications by delaying healing, and have been associated with surgical site and deep prosthetic infections.1, 4
There was also a four-fold decrease (from 8% to 2%) in superficial surgical site complications.1 A further benefit was shown in the significant reduction in the number of total dressing changes required per patient using PICO. Fewer dressing changes may lead to greater patient comfort and less burden on nursing resource. Taking into account reduced LOS for the study group, lower wound complications, lower dressing changes and potential cost savings for wound care in the community due to reduced wound complications in the study group, the authors believes the cost of the PICO is justifiable.1
“Wound complications do not only impact the hospital, but they can have a devastating effect on a patient’s recovery,” explains Helen Griffiths, Outpatient Nurse at RJAH. “There is often a financial impact for the family as more time off work is required, not just for the patient but also their carer. High levels of wound exudate or infections can also cause embarrassment and discomfort, often resulting in significant anxiety. PICO can help prevent these issues through improved wound healing and increased patient confidence.”
While significant benefits have been demonstrated for patients across all demographics, statistical analysis suggests that the use of PICO as a prophylactic for closed surgical incision management in patients that are categorised as high risk (BMI >35, ASA >3 or, diabetics is particularly beneficial.1
The full study is published in Bone and Joint Research in August 2016 and is available on their website.
Mr Sudheer Karlakki will be presenting the results of the RCT at the European Bone and Joint Infection conference in September.
Enquiries
Media
Beth Lowes or Rachel Cunningham
ROAD Communications on behalf of Smith & Nephew
+44 (0)208 995 5832
firstname@roadcommunications.co.uk
About the study
This Investigator Initiated Study (IIS) was performed at Robert Jones and Agnes Hunt Orthopaedic Hospital in which Smith and Nephew Wound Management provided funding and devices, which sought to determine whether the use of a incisional Negative Pressure Wound Therapy (PICO, Smith & Nephew) could give predictable length of stay by improved management of the incisional wound after planned primary joint replacement surgery. This was a 220 patient Randomised Controlled Trial (RCT) of PICO, compared to standard dressings. The PICO group showed an improvement in all areas investigated compared to standard care control group.
About PICO
PICO is cleared for use in hospital and homecare settings in Europe, US, Canada, and Australia. In Japan PICO is cleared for use in open wounds only.For more information about the PICO system and the NPWT portfolio of products from Smith & Nephew, please visit www.smith-nephew.com. Clinicians and patients may also refer to the 24/7 Negative Pressure Wound Therapy hotline, 0800 9155394 (UK) and 1800 303 622 (Ireland)
About Smith & Nephew
Smith & Nephew is a global medical technology business dedicated to helping healthcare professionals improve people’s lives. With leadership positions in Orthopaedic Reconstruction, Advanced Wound Management,Sports Medicine and Trauma & Extremities, Smith & Nephew has around 15,000 employees and a presence in more than 100 countries. Annual sales in 2015 were more than $4.6 billion. Smith & Nephew is a member of the FTSE100 (LSE:SN, NYSE:SNN).
For more information about Smith & Nephew, please visit our website www.smith-nephew.com, follow @SmithNephewplc on Twitter or visit SmithNephewplc on Facebook.com.
Smith & Nephew will be presenting more of the latest research on surgical site complications at WUWHS 2016. To learn more go to http://www.smith-nephew.com/wuwhs2016/.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as “aim”, “plan”, “intend”, “anticipate”, “well-placed”, “believe”, “estimate”, “expect”, “target”, “consider” and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith & Nephew, these factors include: economic and financial conditions in the markets we serve, especially those affecting health care providers, payers and customers; price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers; competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith & Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith & Nephew’s most recent annual report on Form 20-F, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith & Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith & Nephew are qualified by this caution. Smith & Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith & Nephew’s expectations.
◊Trademark of Smith & Nephew.
©July 2016 Smith & Nephew 75112
References