SAN DIEGO – March 14, 2017 – Bioventus, a leader in orthobiologic solutions, today announced its plans for the Annual Meeting of the American Academy of Orthopaedic Surgeons (AAOS) March 13-18 in the San Diego Convention Center. Among the products clinicians visiting Bioventus in booth #5223 can learn more about is GELSYN-3™ the company’s next generation, three-injection hyaluronic acid (HA).
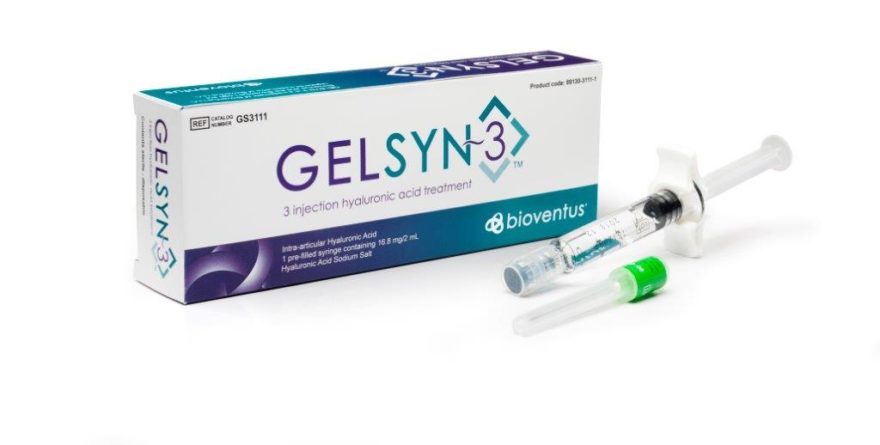
GELSYN-3 helps relieve pain associated with knee osteoarthritis (OA) and is the company’s latest portfolio addition joining its five-injection knee OA solution called SUPARTZ FX®, and its single-injection OA product known as DUROLANE®. Both GELSYN-3 and SUPARTZ FX are available exclusively in the US while DUROLANE is available in OUS markets. Bioventus will also feature the EXOGEN® Ultrasound Bone Healing System and the associated volume of clinical evidence for efficacy that underscores its position as the #1 prescribed bone healing system in the US.
In addition, Bioventus Surgical will highlight its suite of allograft, cell & marrow and synthetic bone graft solutions including: OSTEOAMP®, a uniquely processed allograft bone graft substitute; CELLXTRACT™, a novel cell and bone marrow extraction tool; and SIGNAFUSE™ a bi-phasic mineral composite combined with a patented bioactive glass and resorbable polymer carrier.
“Since it was established in 2012, Bioventus has expanded its portfolio to include 13 offerings for bone healing, osteoarthritis and bone grafts,” said Tony Bihl, CEO, Bioventus. “Our singular and unique focus is on orthobiologics that provide positive outcomes to benefit patients, surgeons, hospitals and payers. We invite AAOS attendees to visit with us and learn more about our solutions which are not only effective, but are backed by clinical and technical data.”
About Bioventus
Bioventus is an orthobiologics company that delivers clinically proven, cost-effective products that help people heal quickly and safely. Its mission is to make a difference by helping patients resume and enjoy active lives. Bioventus has two product portfolios for orthobiologics, Bioventus Active Healing Therapies and Bioventus Surgical that make it a global leader in active orthopaedic healing. Its EXOGEN Ultrasound Bone Healing System is the #1 prescribed bone healing system in the US and is the only FDA-approved bone healing device that uses safe, effective ultrasound to stimulate the body’s natural healing process. Built on a commitment to high quality standards, evidence-based medicine and strong ethical behavior, Bioventus is a trusted partner for physicians worldwide.
For more information, visit www.BioventusGlobal.com and follow the company on Twitter @Bioventusglobal.
Media Contact:
Thomas Hill, +1 919-474-6715, thomas.hill@bioventusglobal.com
Bioventus, the Bioventus logo, DUROLANE, OSTEOAMP and EXOGEN are registered trademarks and GELSYN-3, CELLXTRACT and SIGNAFUSE are trademarks of Bioventus LLC of Bioventus LLC. SUPARTZ FX is a registered trademark of Seikagaku Corp.
Summary of Indications for Use
OSTEOAMP may be used in situations where an autograft is appropriate. It should be restricted to homologous use for the repair, replacement or reconstruction of musculoskeletal defects. Please see OSTEOAMP instructions for use for complete list of contraindications, warnings, and precautions. Full prescribing information can be found in product labeling at BioventusSurgical.com or by contacting customer service at 1-800-637-4391. It is available in the US only.
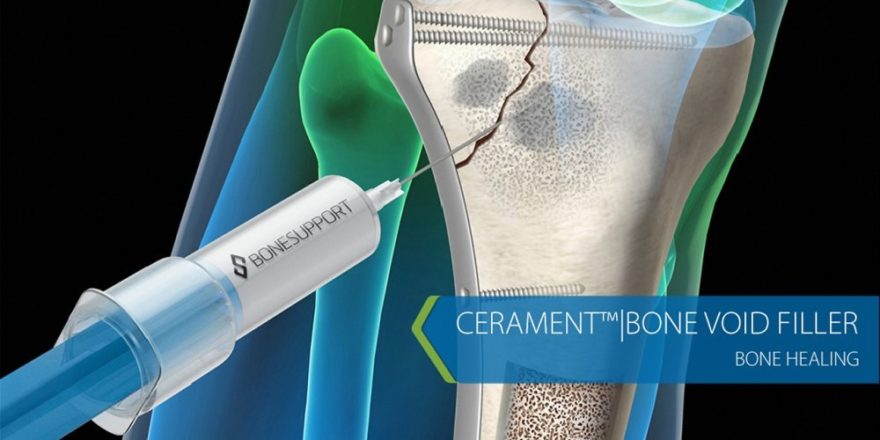
SIGNAFUSE is a bone void filler device intended for use in bony voids or gaps that are not intrinsic to the stability of bony structure. These defects may be surgically created osseous defects or osseous defects created from traumatic injury to the bone. SIGNAFUSE is indicated to be packed gently into bony voids or gaps of the skeletal system (i.e., extremities, pelvis and posterolateral spine fusion procedures). SIGNAFUSE can also be used with autograft as a bone graft extender in the posterolateral spine. The device provides a bone void filler that is resorbed and replaced with host bone during the healing process. Full prescribing information can be found in product labeling at BioventusSurgical.com or by contacting customer service at 1-800-637-4391. It is available in the US only.
CELLXTRACT is intended for use for aspiration of bone marrow or autologous blood using a standard piston syringe. Full prescribing information can be found in product labeling at BioventusSurgical.com by contacting customer service at1-800-637-4391.
SUPARTZ FX is indicated for treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy and simple analgesics, e.g., acetaminophen. You should not use SUPARTZ FX if you have infections or skin diseases at the injection site or allergies to avian (bird) products (feathers and eggs). SUPARTZ FX is not approved for pregnant or nursing women, or children. Risks can include general knee pain, warmth and redness or pain at the injection site. Full prescribing information can be found at www.SupartzFX.com or by contacting customer service at 1-800-836-4080.
GELSYN-3 is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy and simple analgesics (e.g., acetaminophen). GELSYN-3 is not to be administered to patients with known hypersensitivity (allergy) to sodium hyaluronate preparations and should not be injected into the knees of patients having knee joint infections or skin diseases or infections in the area of the injection site. Full prescribing information can be found at www.GelSyn3.com or by contacting customer service at 1-800-836-4080.
EXOGEN
Summary of Indications for Use: In Canada, the EU, Australia and New Zealand
EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of osseous defects (excluding vertebra and skull) including:
- Treatment of delayed union and non-unions†
• Accelerating the time to heal of fresh fractures
• Treatment of stress fractures
• Accelerating repair following osteotomy
• Accelerating repair in bone transport procedures
• Accelerating repair in distraction osteogenesis procedures
• Treatment of joint fusion
† A non-union is considered to be established when the fracture site shows no visibly progressive signs of healing.
There are no known contraindications for the EXOGEN device. Safety and effectiveness have not been established for individuals lacking skeletal maturity, pregnant or nursing women, patients with cardiac pacemakers, on fractures due to bone cancer, or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel. Full prescribing information can be found in product labeling at www.exogen.com.
Summary of Indications for Use in the US
*Summary of Indications for Use: The EXOGEN Ultrasound Bone Healing System is indicated for the non-invasive treatment of established non-unions* excluding skull and vertebra. In addition, EXOGEN is indicated for accelerating the time to a healed fracture for fresh, closed, posteriorly displaced distal radius fractures and fresh, closed or Grade I open tibial diaphysis fractures in skeletally mature individuals when these fractures are orthopaedically managed by closed reduction and cast immobilization.
There are no known contraindications for the EXOGEN device. Safety and effectiveness has not been established for individuals lacking skeletal maturity; pregnant or nursing women; patients with cardiac pacemakers; on fractures due to bone cancer; or on patients with poor blood circulation or clotting problems. Some patients may be sensitive to the ultrasound gel. Full prescribing information can be found in product labeling, at www.exogen.com or by contacting customer service at 1-800-836-4080.
*A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
DUROLANE
European Union and Chile:
DUROLANE (3ml): Symptomatic treatment of mild to moderate knee or hip osteoarthritis. In addition, DUROLANE has been approved in the EU for the symptomatic treatment associated with mild to moderate osteoarthritis pain in the ankle, shoulder, elbow, wrist, fingers, and toes.
DUROLANE SJ (1ml): Symptomatic treatment associated with mild to moderate osteoarthritis pain in the ankle, elbow, wrist, fingers, and toes.
Both DUROLANE and DUROLANE SJ are also indicated for pain following joint arthroscopy in the presence of osteoarthritis within 3months of the procedure.
Full prescribing information can be found in product labeling, or at www.durolane.com.
Canada:
DUROLANE (3ml): Symptomatic treatment of mild to moderate knee or hip osteoarthritis. In addition, DUROLANE has been licensed for the symptomatic treatment associated with mild to moderate osteoarthritis pain in the ankle, fingers and toes.
DUROLANE SJ (1ml): Symptomatic treatment associated with mild to moderate osteoarthritis pain in the ankle, fingers and toes.
Both DUROLANE and DUROLANE SJ are also indicated for pain following joint arthroscopy in the presence of osteoarthritis within 3months of the procedure. There are no known contraindications. You should not use DUROLANE if you have infections or skin disease at the injection site. DUROLANE has not been tested in pregnant or lactating women, or children. Risks can include transient pain, swelling and/or stiffness at the injection site.
Full prescribing information can be found in product labeling, or at www.durolane.com.
Australia/New Zealand/Mexico:
DUROLANE (3ml): Symptomatic treatment of mild to moderate knee osteoarthritis.
There are no known contraindications. You should not use DUROLANE if you have infections or skin disease at the injection site. DUROLANE has not been tested in pregnant or lactating women, or children. Risks can include transient pain, swelling and/or stiffness at the injection site. Full prescribing information can be found in product labeling, or at www.durolane.com.
India, Indonesia, U.A.E., Saudi Arabia, Jordan, Hong Kong, Russia:
DUROLANE (3ml): Symptomatic treatment of mild to moderate knee and hip osteoarthritis.
There are no known contraindications. You should not use DUROLANE if you have infections or skin disease at the injection site. DUROLANE has not been tested in pregnant or lactating women, or children. Risks can include transient pain, swelling and/or stiffness at the injection site. Full prescribing information can be found in product labeling, or at www.durolane.com.
Taiwan:
DUROLANE (3ml): Treatment of pain in OA of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy and simple analgesics, e.g., acetaminophen.
There are no known contraindications. You should not use DUROLANE if you have infections or skin disease at the injection site. DUROLANE has not been tested in pregnant or lactating women, or children. Risks can include transient pain, swelling and/or stiffness at the injection site. Full prescribing information can be found in product labeling, or at www.durolane.com.