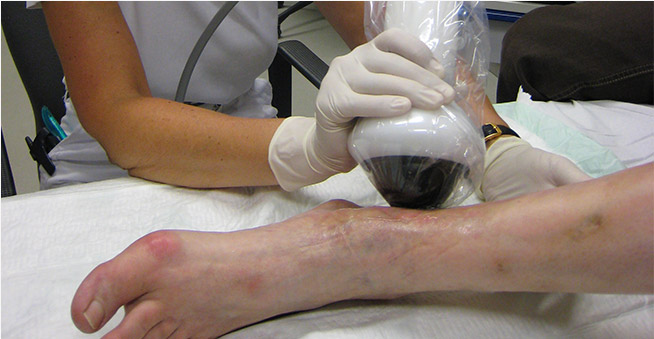
SUWANEE, GA, Jan. 02, 2018 (GLOBE NEWSWIRE) — SANUWAVE Health, Inc. (OTCQB: SNWV) has announced that the U.S. Food and Drug Administration (FDA) has issued its decision on the de novo submission for the dermaPACE® System. Their decision, dated December 28, 2017, permits the marketing of the dermaPACE System as a Class II medical device used for the treatment of Diabetic Foot Ulcers (DFU) in the U.S., the world’s largest medical device market. This order by FDA is the culmination of intensive clinical studies by SANUWAVE and diligent work by both SANUWAVE employees and our regulatory partners, Musculoskeletal Clinical Regulatory Advisers (MCRA) in successfully submitting documentation and interacting with FDA during the clearance process.
The dermaPACE system was evaluated using two studies under an FDA approved IDE. The studies were designed as prospective, randomized, double-blind, parallel-group, sham-controlled, multi-center 24-week studies at 39 centers. A total of 336 subjects were enrolled and treated with either active dermaPACE plus conventional therapy or sham dermaPACE plus conventional therapy (a.k.a. standard of care). Conventional therapy included, but was not limited to, debridement, saline-moistened gauze, and pressure reducing footwear. The objective of the studies was to compare the safety and efficacy of the dermaPACE System to sham-control application. The prospectively defined primary efficacy endpoint for the dermaPACE System studies was the incidence of complete wound closure at 12 weeks post-initial application of the dermaPACE system (active or sham). Complete wound closure was defined as complete skin re-epithelialization without drainage or dressing requirements, confirmed over two consecutive visits within 12-weeks. If the wound was considered closed for the first time at the 12-week visit, then the next visit was used to confirm closure. Investigators continued to follow subjects and evaluate wound closure through 24 weeks.
The patients who were treated with the dermaPACE System showed an increase in wound healing at 24 weeks with a 44 percent wound closure rate. Those patients treated with the sham shock wave therapy showed a 30 percent wound closure rate during the same time period. Unlike other advanced DFU treatment modalities, the dermaPACE protocol did not allow any form of closure by secondary intent (e.g. sutures). The wound closures in the dermaPACE arm were robust with less than 10% rates of recurrence. In addition, the rate of wound area reduction in the dermaPACE cohort was significant compared to that of the Sham arm beginning at six weeks and continuing on through the remainder of the 24 treatment and follow-up phases.
The dermaPACE System offers a novel treatment modality for DFUs, delivering shock wave energy in a non-invasive manner. Treatment consists of 4 – 8 short, non-invasive applications over a 2 to 10 week period. Monitoring and standard of care is required thereafter. This non-invasive treatment provides lower patient adverse events compared with other procedures like amputation or skin grafting, and as a result, the dermaPACE System provides a safe and effective option for treating patients with diabetic foot ulcers.
There are many different modalities, ranging from simple dressings, to biologics, matrices, pharmaceuticals, and devices. Existing therapies require either:
continuous application of a pharmacologic agent;
- repeated application of a skin equivalent (up to 8 times over a 12 week period);
- repeated implantation of a dermal substitute (weekly, up to 8 applications and after a 6 week run-in); or
- the attachment of a vacuum assisted device to the wound area for prolonged periods of time.
SANUWAVE’s Chairman of the Board and CEO, Kevin A. Richardson II said, “This regulatory clearance marks the biggest milestone yet for SANUWAVE, and the Company is poised to commence the commercial rollout in the United States shortly.” The dermaPACE System is currently available in the European Union, South Korea, Canada, Australia, and New Zealand and is under regulatory review in Taiwan and Indonesia. SANUWAVE has just entered into a major distribution agreement in Brazil, where device approval is anticipated later in 2018.
According to American Diabetes Association statistics from 2015, 30.3 million Americans, or 9.4% of the population, had diabetes. And the annual cost of diabetes, was $245 billion in 2012. This figure comprises $176 billion in excess healthcare expenditures and $69 billion in reduced workforce productivity. Approximately 15% of diabetic patients will develop lower extremity ulcers and 14 – 24% of DFUs will eventually undergo amputation. “The market opportunity for the dermaPACE System in the U.S. is significant. As U.S. diabetes rates continue to rise and growing numbers of diabetics develop DFUs, we are confident dermaPACE will positively redefine the treatment of diabetic foot ulcers in the U.S.,” said Mr. Richardson.
In addition to causing suffering and morbidity, foot lesions in diabetic patients have substantial economic consequences. Diabetic foot complications result in huge costs for both society and the individual patients. The economic burden of DFUs and the complications arising from them are enormous. The cost to treat a DFU over a 2-year period was $27,987 in 1995 and, based on the medical component of the US Consumer Price Index, rose to $46,841 in 2009. These high costs have been linked to frequent outpatient appointments, in-patient days, laboratory tests, drugs/medications, hospital stays, and secondary complications of osteomyelitis and amputation. Direct costs for a lower-extremity amputation range from $22,700 to $51,300.
Mr. Richardson continues, “Due to its non-invasive nature, the dermaPACE System does not prevent the use of other treatment options (i.e., dermal grafts, wound matrices, biologic gels, and surgery) in conjunction with the dermaPACE System or in later stages of wound care. In fact, wound care professionals using the device in Europe, Canada, and Australia use the dermaPACE System in the progression of their treatment regimen for wound management with healing as the final intent. Not all wounds, nor all patients are the same; therefore, each wound is treated on an individual basis based on the treating physician’s knowledge and the treatment options available. The dermaPACE system offers the ability to manage the DFUs chronicity and is a powerful tool in the clinician’s arsenal to achieve wound closure.”
U.S. Commercial Launch
SANUWAVE has engaged in a range of preparatory activities to ensure it is ready for an imminent U.S. market release, including identifying and hiring the first tier of sales personnel; dialogue with targeted hospitals for initial adoption; and conducting early industry and patient awareness initiatives.
“With the FDA de novo order in place, we will now accelerate our U.S. sales process with strategic partnerships and the hiring of key sales personnel to begin initial, targeted market release in the U.S” said Mr. Richardson. “SANUWAVE is excited to advance our efforts to bring the dermaPACE System to the U.S. market.”
Our Regulatory Partners
In January 2015 SANUWAVE retained Musculoskeletal Clinical Regulatory Advisers, LLC (MCRA) to lead the Company’s interactions and correspondence with the FDA for the dermaPACE. MCRA has successfully worked with the FDA on numerous PMAs for various musculoskeletal, restorative and general surgical devices since 2004. At the time, SANUWAVE had withdrawn a PMA submitted using data from the first DFU clinical study. At FDA’s request, SANUWAVE initiated a supplemental DFU study. SANUWAVE recognized the need to pull in MCRA’s expertise to help navigate uncharted regulatory pathways.
Glenn Stiegman, MCRA’s Vice President of Clinical and Regulatory Affairs, explained, “MCRA was intrigued by SANUWAVE’s position. Their studies had compelling clinical data showing that the dermaPACE System was safe and effective, and SANUWAVE’s situation presented a unique but solvable challenge. We are pleased SANUWAVE asked us to work with them to develop and execute an FDA response strategy and assist in leading the dermaPACE to clearance.”
Mr. Richardson added, “We chose MCRA for their extensive regulatory experience and interaction with FDA over the past decade. They took data from, essentially, a Not-Approvable PMA, and worked wonders in obtaining a de novo decision with basically the same data. Their depth of knowledge and experience in regulatory and clinical science made them an excellent partner for working hand-in-hand with our team and the FDA.”
About SANUWAVE Health, Inc.
SANUWAVE Health, Inc. (OTCQB:SNWV) (www.sanuwave.com) is a shock wave technology company initially focused on the development and commercialization of patented noninvasive, biological response activating devices for the repair and regeneration of skin, musculoskeletal tissue and vascular structures. SANUWAVE’s portfolio of regenerative medicine products and product candidates activate biologic signaling and angiogenic responses, producing new vascularization and microcirculatory improvement, which helps restore the body’s normal healing processes and regeneration. SANUWAVE applies its patented PACE technology in wound healing, orthopedic/spine, plastic/cosmetic and cardiac conditions. Its lead product candidate for the global wound care market, dermaPACE, is CE Marked throughout Europe and has device license approval for the treatment of the skin and subcutaneous soft tissue in Canada, Australia and New Zealand. In the U.S., dermaPACE System has been approved under the FDA’s de novo petition process for the treatment of diabetic foot ulcers. SANUWAVE researches, designs, manufactures, markets and services its products worldwide, and believes it has demonstrated that its technology is safe and effective in stimulating healing in chronic conditions of the foot (plantar fasciitis) and the elbow (lateral epicondylitis) through its U.S. Class III PMA approved OssaTron® device, as well as stimulating bone and chronic tendonitis regeneration in the musculoskeletal environment through the utilization of its OssaTron, Evotron® and orthoPACE devices in Europe, Asia and Asia/Pacific. In addition, there are license/partnership opportunities for SANUWAVE’s shock wave technology for non-medical uses, including energy, water, food and industrial markets.
About MUSCULOSKELETAL CLINICAL REGULATORY ADVISERS, LLC (MCRA)
Founded in 2004, Musculoskeletal Clinical Regulatory Advisers, LLC (MCRA) is a leading adviser and clinical research organization to the neuro-musculoskeletal and medical device industries. MCRA’s value lies in its industry experience and integration of five business value creators: regulatory, reimbursement, clinical research, healthcare compliance and quality assurance. MCRA’s integrated approach of these key value creating initiatives provides unparalleled expertise for its clients. MCRA has offices in Washington, DC, Manchester, CT and New York, NY, and serves over 500 clients globally.
Forward-Looking Statements
This press release may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, such as statements relating to financial results and plans for future business development activities, and are thus prospective. Forward-looking statements include all statements that are not statements of historical fact regarding intent, belief or current expectations of the Company, its directors or its officers. Investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, many of which are beyond the Company’s ability to control. Actual results may differ materially from those projected in the forward-looking statements. Among the key risks, assumptions and factors that may affect operating results, performance and financial condition are risks associated with the regulatory approval and marketing of the Company’s product candidates and products, unproven pre-clinical and clinical development activities, regulatory oversight, the Company’s ability to manage its capital resource issues, competition, and the other factors discussed in detail in the Company’s periodic filings with the Securities and Exchange Commission. The Company undertakes no obligation to update any forward-looking statement.
For additional information about the Company, visit www.sanuwave.com.
Contact:
Millennium Park Capital LLC
Christopher Wynne
312-724-7845
cwynne@mparkcm.com
SANUWAVE Health, Inc.
Kevin Richardson II
Chairman of the Board
978-922-2447
investorrelations@sanuwave.com
![]()