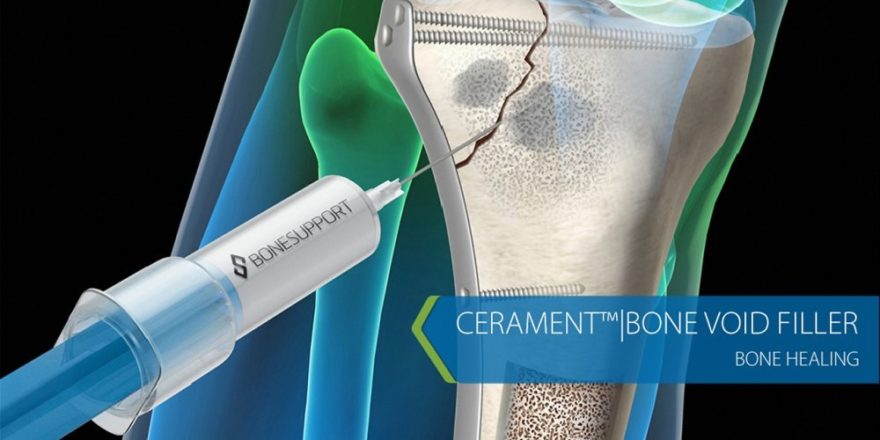
Lund, Sweden, 14 March, 2017 – BONESUPPORT AB, an emerging leader in innovative injectable bioceramic bone scaffolds to treat bone voids caused by trauma, infection, disease or related surgery, announces that it has extended the term of its U.S. distribution agreement with Zimmer Biomet. Under the agreement, Zimmer Biomet will continue to have exclusive rights for BONESUPPORT’s proprietary CERAMENT BONE VOID FILLER product line for Orthopedics, Trauma and Foot and Ankle indications in the United States.
“We are extremely pleased to extend our distribution agreement with Zimmer Biomet,” said Richard Davies, CEO of BONESUPPORT™. “The partnership has been very successful and has resulted in the current rapid growth of our flagship product, CERAMENT in the world’s largest bone graft substitute market. This rapid growth is building an important platform from which we can launch product extensions into the US.”
In addition to commercialization of CERAMENT BONE VOID FILLER in the U.S. market, BONESUPPORT is currently enrolling patients into the FORTIFY Clinical Study, an FDA approved IDE randomized control pivotal study for the Company’s anti-biotic eluting product CERAMENT G. CERAMENT G is currently approved and commercialized in the EU and other markets outside the United States.
About BONESUPPORT™
BONESUPPORT has developed CERAMENT as an innovative range of radiopaque injectable osteoconductive bioceramic products that have a proven ability to heal defects by remodeling to host bone in six to 12 months. Our products are effective in treating patients with fractures and bone voids caused by trauma, infection, disease or related surgery. Our lead product, CERAMENT BONE VOID FILLER (BVF) addresses important issues facing health care providers, such as avoiding hospital readmissions and revision surgery that result from failed bone healing and infection caused by residual bone voids. CERAMENT BVF is commercially available in the U.S., EU, SE Asia and the Middle East.
CERAMENT’s distinctive properties as a drug eluting material have been validated in clinical practice by CERAMENT G and CERAMENT V, the first CE-marked injectable antibiotic eluting bone graft substitutes. These products provide local sustained delivery of gentamicin and vancomycin, respectively. The local delivery feature enables an initial high concentration of antibiotics to the bone defect and then a longer sustainable dose above the minimal inhibitory concentration (MIC) to protect bone healing and promote bone remodeling.
CERAMENT G and CERAMENT V have demonstrated good results in patients with problematic bone infections including osteomyelitis. They are also used prophylactically in patients who are at risk for developing infection. CERAMENT G and CERAMENT V are available in the EU.
BONESUPPORT was founded in 1999 by Prof. Lars Lidgren, an internationally respected scientist who has been the President of various musculoskeletal societies. BONESUPPORT’s mission is to improve the lives of patients suffering from bone disorders that cause bone voids, lead to injury, breakage, pain, and reduced quality of life. The Company is based in Lund, Sweden. www.bonesupport.com
BONESUPPORT and CERAMENT are registered trademarks.
Contact Information
Citigate Dewe Rogerson
David Dible, Andrea Bici, Mark Swallow
+44 (0)20 7282 2949/1050/2948