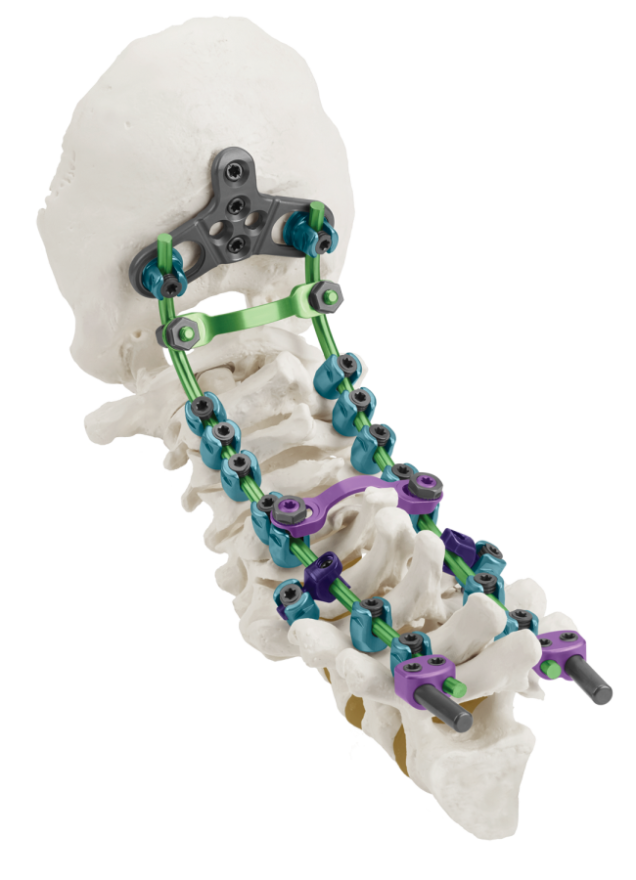
AUDUBON, Pa., Oct. 25, 2016 (GLOBE NEWSWIRE) — Globus Medical, Inc. (NYSE:GMED), a leading musculoskeletal implant manufacturer, continues to innovate in the treatment of complex spinal instability and deformity with today’s announcement of the launch of the QUARTEX™ OCT Stabilization System.
QUARTEX™ offers a variety of solutions to the challenges associated with posterior OCT fusion while delivering paramount reliability and ease of use. QUARTEX™ screw heads accept 3.5mm or 4.0mm rods in either titanium or cobalt chrome alloy and offer 90° of conical angulation. QUARTEX™ allows surgeons to take full advantage of thoracic anatomy through the use of larger screws with diameters up to 5.5mm. Refined instruments facilitate construct assembly with efficient reduction options and intuitive screwdrivers.
“QUARTEX™ provides a single, seamless solution to posterior OCT fixation,” said Chad Glerum, Director of Fixation, Product Development. “Our design team of engineers and spine surgeons scrutinized every aspect of the implants and instruments to optimize flow and functionality. We are excited to see QUARTEX™ provide a versatile OCT solution to propel Globus’ continued growth in this market.”
Dr. Richard Frisch added, “I’m excited that I can choose a 4.0mm rod for my longer constructs and larger diameter screws in the upper thoracic pedicles. The increased stiffness and bone purchase gives me a lot of confidence in the stability of the construct. The drivers provide firm attachment to the screws and the threaded locking caps engage easily, which help my cases run smoothly. QUARTEX™ is the most versatile system I have used and is a great addition to my practice.”
Indications
The QUARTEX™ Occipito-Cervico-Thoracic Spinal System implants are intended to provide immobilization and stabilization of spinal segments as an adjunct to fusion for the following acute and chronic instabilities of the craniocervical junction, the cervical spine (C1-C7) and the thoracic spine (T1-T3): traumatic spinal fractures and/or traumatic dislocations; instability or deformity; failed previous fusions (e.g. pseudoarthrosis); tumors involving the cervical/thoracic spine; and degenerative disease, including intractable radiculopathy and/ or myelopathy, neck and/or arm pain of discogenic origin as confirmed by radiographic studies, and degenerative disease of the facets with instability. These implants are also intended to restore the integrity of the spinal column even in the absence of fusion for a limited time period in patients with advanced stage tumors involving the cervical spine in whom life expectancy is of insufficient duration to permit achievement of fusion. In order to achieve additional levels of fixation, rods may be connected to occipital cervical thoracic or thoracolumbar stabilization systems ranging in diameter from 3.2mm to 6.5mm, using corresponding connectors.
About Globus Medical, Inc.
Globus Medical, Inc. is a leading musculoskeletal implant company based in Audubon, PA. The company was founded in 2003 by an experienced team of professionals with a shared vision to create products that enable surgeons to promote healing in patients with musculoskeletal disorders. Additional information can be accessed at http://www.globusmedical.com.
Safe Harbor Statements
All statements included in this press release other than statements of historical fact are forward-looking statements and may be identified by their use of words such as “believe,” “may,” “might,” “could,” “will,” “aim,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “plan” and other similar terms. These forward-looking statements are based on our current assumptions, expectations and estimates of future events and trends. Forward-looking statements are only predictions and are subject to many risks, uncertainties and other factors that may affect our businesses and operations and could cause actual results to differ materially from those predicted. These risks and uncertainties include, but are not limited to, factors affecting our quarterly results, our ability to manage our growth, our ability to sustain our profitability, demand for our products, our ability to compete successfully (including without limitation our ability to convince surgeons to use our products and our ability to attract and retain sales and other personnel), our ability to rapidly develop and introduce new products, our ability to develop and execute on successful business strategies, our ability to comply with changing laws and regulations that are applicable to our businesses, our ability to safeguard our intellectual property, our success in defending legal proceedings brought against us, trends in the medical device industry, general economic conditions, and other risks. For a discussion of these and other risks, uncertainties and other factors that could affect our results, you should refer to the disclosure contained in our most recent annual report on Form 10-K filed with the Securities and Exchange Commission, including the sections labeled “Risk Factors” and “Cautionary Note Concerning Forward-Looking Statements,” and in our Forms 10-Q, Forms 8-K and other filings with the Securities and Exchange Commission. These documents are available at www.sec.gov. Moreover, we operate in an evolving environment. New risk factors and uncertainties emerge from time to time and it is not possible for us to predict all risk factors and uncertainties, nor can we assess the impact of all factors on its business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. Given these risks and uncertainties, readers are cautioned not to place undue reliance on any forward-looking statements. Forward-looking statements contained in this press release speak only as of the date of this press release. We undertake no obligation to update any forward-looking statements as a result of new information, events or circumstances or other factors arising or coming to our attention after the date hereof.
Contact: Dan Scavilla Senior Vice President and Chief Financial Officer Phone: (610) 930-1800 Email: investors@globusmedical.com www.globusmedical.com