WARSAW, Ind., Feb. 7, 2017 /PRNewswire/ — Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global leader in musculoskeletal healthcare, announced today the international release of the Subchondroplasty® (SCP®) Procedure. The Company received CE Mark approval for the commercialization of its SCP Procedure to facilitate distribution in the European Union (EU) and other countries that recognize the CE Mark. Zimmer Biomet also received approval for distribution in Canada, Singapore, Malaysia and Hong Kong.
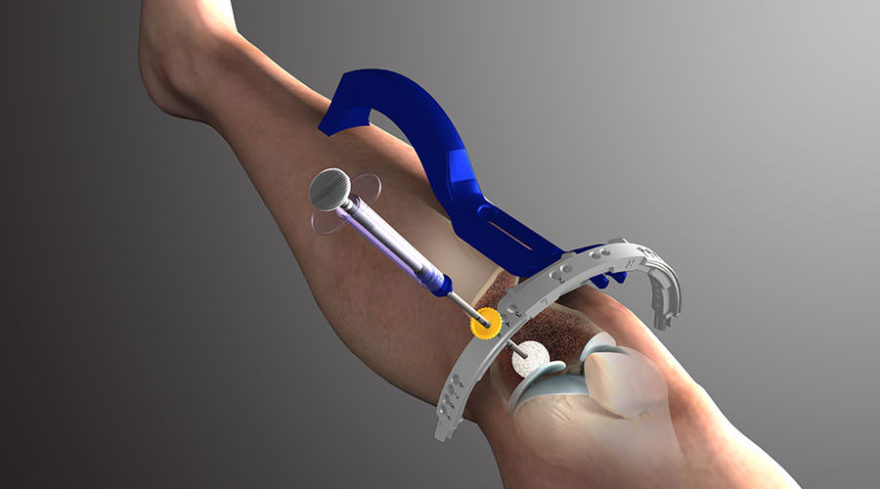
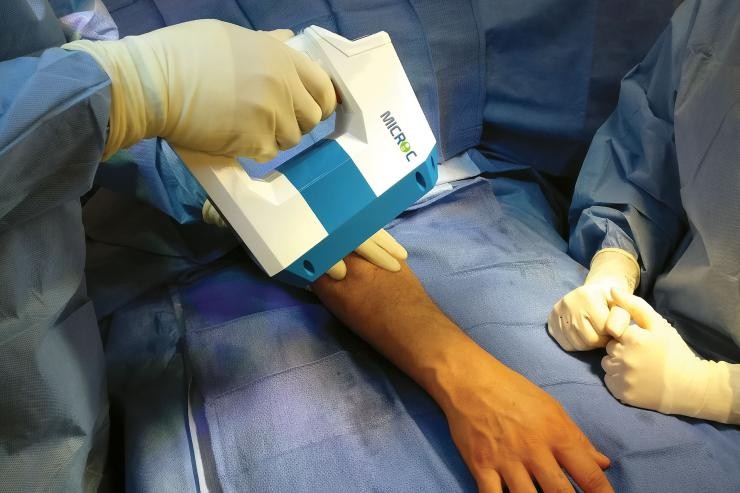
The SCP Procedure is a minimally invasive outpatient intervention that addresses the defects associated with subchondral bone marrow lesions (BMLs). Diagnosed using an MRI and physical exam, BMLs are associated with stress fractures or micro-fractures of the bone adjacent to the joint. Left untreated, these defects may lead to cartilage degeneration, limited function, pain and a greater risk for joint deterioration.
SCP is performed to repair chronic BMLs by filling them with AccuFill® Bone Substitute Material, a porous injectable calcium phosphate (CaP). The bone substitute is then slowly resorbed and replaced with healthy bone, repairing the bone defect.
The procedure is usually performed along with arthroscopy for visualization and treatment of findings inside the joint. In some cases, an open or mini-open procedure is necessary for access to the defect.
“The Subchondroplasty procedure addresses a previously unmet need in my practice for patients with chronic and painful bone marrow lesions,” said Dr. Christopher Baker, orthopaedic surgeon at Florida Orthopedic Institute in Tampa, Fla. “These patients no longer benefit from conservative treatment, yet are not ready for total joint replacement surgery.”
Initially used only in bone defects of the knee, the SCP Procedure has been successfully performed in other areas including bones of the foot, ankle and hip. Zimmer Biomet now owns 36 patents and eight trademarks in this area, with numerous others still pending.
“The international release of the Subchondroplasty Procedure is a major milestone for our company and for patients with chronic bone marrow lesions,” said David Nolan, Zimmer Biomet Group President, Biologics, Extremities, Sports Medicine, Surgical, Trauma, Foot and Ankle, Office Based Technologies and Zimmer Biomet Signature Solutions. “The procedure offers a tool that fills a gap in the patient treatment algorithm for the surgeon. We are eager to begin our international commercial launch.”
For more information on the SCP Procedure, please visit www.subchondroplasty.com.
About Zimmer Biomet
Founded in 1927 and headquartered in Warsaw, Indiana, Zimmer Biomet is a global leader in musculoskeletal healthcare. We design, manufacture and market orthopaedic reconstructive products; sports medicine, biologics, extremities and trauma products; office based technologies; spine, craniomaxillofacial and thoracic products; dental implants; and related surgical products.
We collaborate with healthcare professionals around the globe to advance the pace of innovation. Our products and solutions help treat patients suffering from disorders of, or injuries to, bones, joints or supporting soft tissues. Together with healthcare professionals, we help millions of people live better lives.
We have operations in more than 25 countries around the world and sell products in more than 100 countries. For more information, visit www.zimmerbiomet.com or follow Zimmer Biomet on Twitter at www.twitter.com/zimmerbiomet.
Cautionary Statement Regarding Forward-Looking Statements
This news release contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, but are not limited to, statements concerning Zimmer Biomet’s expectations, plans, prospects, and product and service offerings, including new product launches and potential clinical successes. Such statements are based upon the current beliefs and expectations of management and are subject to significant risks and uncertainties that could cause actual outcomes and results to differ materially. For a list and description of some of such risks and uncertainties, see our periodic reports filed with the SEC. These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in Zimmer Biomet’s filings with the SEC. We disclaim any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be set forth in our periodic reports. Accordingly, such forward-looking statements speak only as of the date made. Readers of this news release are cautioned not to place undue reliance on these forward-looking statements, since, while management believes the assumptions on which the forward-looking statements are based are reasonable, there can be no assurance that these forward-looking statements will prove to be accurate. This cautionary statement is applicable to all forward-looking statements contained in this news release.
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/zimmer-biomet-announces-the-international-release-of-the-innovative-subchondroplasty-procedure-300403563.html
SOURCE Zimmer Biomet Holdings, Inc.
News Provided by Acquire Media