FRANKLIN, Mass., Sept. 12, 2017 /PRNewswire/ — Arthrosurface, Inc. announced today the publication of long-term HemiCAP® data in The Journal of Foot and Ankle Surgery, “10 Year Follow-Up of Metatarsal Head Resurfacing Implants for Treatment of Hallux Rigidus.” The study reported excellent pain relief, functional improvement, high patient satisfaction, a low reoperation rate and no serious complications.
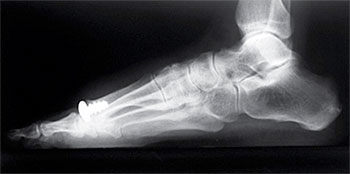
Hallux Rigidus describes a painful condition which affects the big toe at the first metatarsal joint. Osteophytes, or bone spurs, develop on the dorsal surface of the bones involved, which limit motion and cause pain. Advanced disease may also include degenerate cartilage on the joint surfaces, which can contribute to patient pain.
Historically, arthrodesis, or joint fusion, was the standard of care to address patients’ symptoms in advanced stages, however, with eliminated joint motion, patients have shown difficulties with rising on their toes, kneeling or taking a full stride when walking. Other complications include non-union of the fusion site in about 10% of the procedures.
The proprietary HemiCAP® Implant System is designed to maximize implant stability through a strong threaded fixation component and optimizes the fit with a range of implant curvatures. As an active alternative to joint fusion, the HemiCAP® System allows physicians to provide patient specific solutions with hemiarthroplasty of the first and lesser toes, as well as total joint replacement of the big toe. The motion preserving platform provides a complete solution for primary and revision surgery, while maintaining an exit into joint fusion. To date more than 33,000 implants have been used.
“Patients today want to maintain their toe mobility to stay active, whether it is playing golf, performing yoga or being able to continue to live independently,” said Lisa Donnelly VP of Marketing. “We see tremendous traffic on our website from patients inquiring how to find a physician who is trained on our technologies. We have recently launched a new physician locator covering a nationwide network of approximately 1,000 foot and ankle surgeons to help assist them.”
“It is very satisfying to see the AOFAS score of 90.6 at the 10-year follow-up and the high degree of patient satisfaction,” said Steve Ek, President & CEO of Arthrosurface. “We hope this data continues to drive the clinical and patient communities to question the role of motion sacrificing procedures in the first MTP joint, as has happened over time in multiple other joints”.
About Arthrosurface
Arthrosurface, Inc. is a global orthopedic medical technology business providing a broad portfolio of essential products and instrumentation used to treat upper and lower extremity orthopedic conditions caused by trauma, injury and arthritic disease. The product offerings include devices, instruments and orthobiologics designed to preserve and restore the joints so patients can regain and maintain an active lifestyle. The Company offers a variety of unique systems that provide less invasive technologies for surgeons that can be used to treat a wide range of joint conditions. Founded in 2002, Arthrosurface markets and distributes its products in the US and around the world and has succeeded in helping patients return to activity for over 13 years. For more information, please go to our website at www.arthrosurface.com
SOURCE Arthrosurface, Inc.