July 16, 2018
ALPHARETTA, Ga.–(BUSINESS WIRE)–Cartiva, Inc., (Company) a medical device company focused on the treatment of osteoarthritis of the extremities, announced today the presentation of five-year follow up results from its pivotal MOTION Study, a prospective, randomized multi-center study which evaluated the safety and effectiveness of its Cartiva Synthetic Cartilage Implant (SCI) for the treatment of arthritis at the base of the big toe, the most common site of arthritis in the foot. The objective of the MOTION study, which involved 197 patients treated at 12 centers, was to establish non-inferiority of Cartiva SCI compared to fusion, the historical standard of care.
Judith Baumhauer, M.D., M.P.H., Professor and Associate Chair of Academic Affairs, University of Rochester Medical Center and Principal Investigator of the MOTION Study, presented the data July 14 at the annual meeting of the American Orthopaedic Foot and Ankle Society (AOFAS) in Boston, MA.
In the original MOTION Study, 135 patients received the Cartiva implant. Of these, long-term outcomes were available for 106 patients with a mean follow-up of 5.8 ± 0.7 (range: 4.4 to 8.0) years. These outcomes demonstrate that Cartiva SCI provides:
- Durable pain relief, with patients achieving a 97% median reduction in pain.
- Sustainable functional improvement, with patients demonstrating a 176% median improvement in sporting activities.
- Motion preservation, with patients experiencing a 25% improvement in range of motion from baseline.
- A high rate of satisfaction with the treatment, with 93% of patients saying they would have the procedure again.
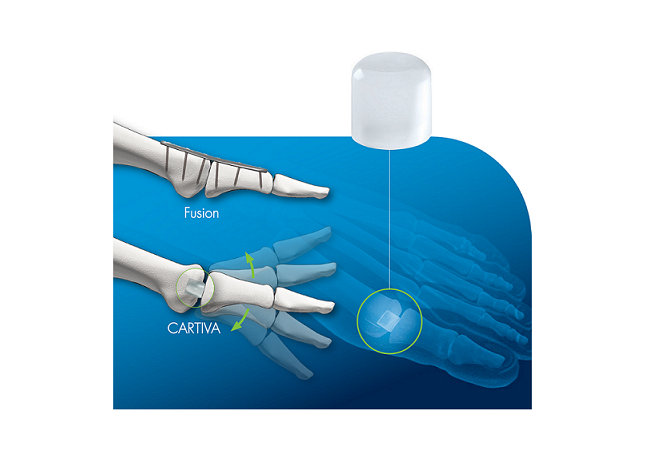
“Cartiva SCI has now been proven to be a viable alternative to fusion for patients wanting to maintain range of motion,” said Mark Glazebrook, MD, FRCSC, MSc, PhD, Professor of Orthopaedics Surgery at Queen Elizabeth Sciences Centre in Nova Scotia, Canada and a lead enroller in the MOTION Study. “With almost six years of follow up from a rigorously conducted clinical trial, the data supports Cartiva being a game-changer in the treatment of this condition.”
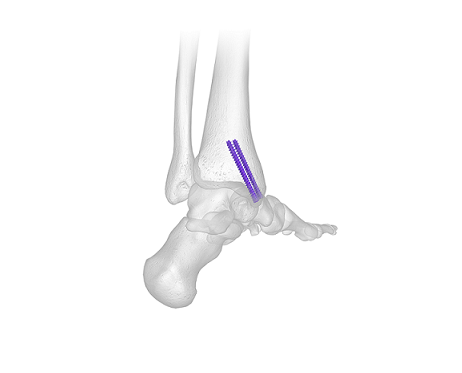
Cartiva SCI gained Food and Drug Administration (FDA) premarket approval for use in arthritis at the base of the big toe in July 2016. As a condition to the approval, FDA required additional data to evaluate the long-term safety and effectiveness of Cartiva SCI, including the durability and survivorship of the implant. The primary endpoint (implant survivorship) and secondary endpoints (clinical and radiographic success for the Cartiva subjects through five years) were met and the data has been submitted to FDA.
“We were very excited to share the long-term results with the attendees of the AOFAS Annual Meeting,” said Tim Patrick, President and CEO of Cartiva. The excellent follow-up out to as long as eight years is a testament to our clinical investigators and their research staff. We believe these data support Cartiva as the standard of care for a motion-preserving alternative to fusion.”
About Cartiva, Inc.
Based in Alpharetta, Ga., Cartiva, Inc. is a medical device company focused on the treatment of osteoarthritis of the extremities. Cartiva’s synthetic cartilage is the only FDA-approved biomedical implant that mimics natural cartilage. The company’s venture investors include New Enterprise Associates and Windham Venture Partners. Additional information is available on the company’s website at www.cartiva.net.
Contacts
Cartiva, Inc.
Peter Pizzo, 770-754-3855
Chief Financial Officer