February 6, 2018 – Authors:
ABSTRACT
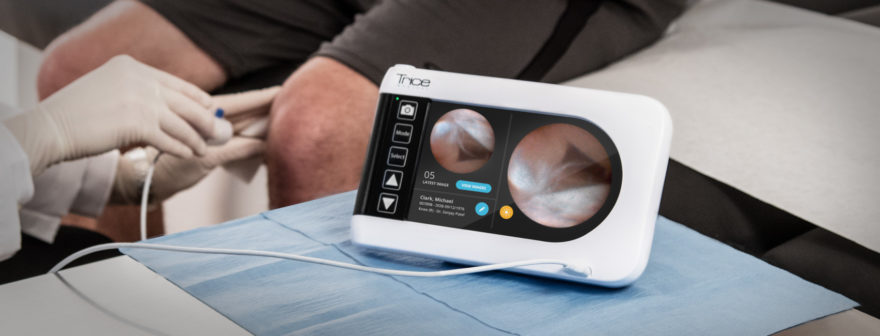
The use of arthroscopy for purely diagnostic purposes has been largely supplanted by noninvasive technologies, such as magnetic resonance imaging (MRI). The mi-eye+TM (Trice Medical) technology is a small-bore needle unit for in-office arthroscopy. We conducted a pilot study comparing the mi-eye+TM unit with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to MRI for the diagnosis of intra-articular pathology of the knee.
This prospective, multicenter, observational study was approved by the Institutional Review Board. There were 106 patients (53 males, 53 females) in the study. MRIs were interpreted by musculoskeletally trained radiologists. The study was conducted in the operating room using the mi-eye+TM device. The mi-eye+ TM device findings were compared with the MRI findings within individual pathologies, and a “per-patient” analysis was performed to compare the arthroscopic findings with those of the mi-eye+TM and the MRI. Additionally, we identified all mi-eye+TM findings and MRI findings that exactly matched the surgical arthroscopy findings.
The mi-eye+TM demonstrated complete accuracy of all pathologies for 97 (91.5%) of the 106 patients included in the study, whereas MRI demonstrated complete accuracy for 65 patients (61.3%) (P < .0001). All discrepancies between mi-eye+TM and arthroscopy were false-negative mi-eye+TM results, as the mi-eye+TM did not reveal some aspect of the knee’s pathology for 9 patients. The mi-eye+TM was more sensitive than MRI in identifying meniscal tears (92.6% vs 77.8%; P = .0035) and more specific in diagnosing these tears (100% vs 41.7%; P < .0001).
The mi-eye+TM device proved to be more sensitive and specific than MRI for intra-articular findings at time of knee arthroscopy. Certainly there are contraindications to using the mi-eye+TM, and our results do not obviate the need for MRI, but our study did demonstrate that the mi-eye+TM needle arthroscope can safely provide excellent visualization of intra-articular knee pathology.
In the early 1990s, in-office needle arthroscopy was described as a viable means of diagnosing pathologies and obtaining synovial biopsies from the knee.1-3 Initial results were good, and the procedures had very low complication rates. Nevertheless, in-office arthroscopy of the knee is not yet widely performed, likely given concerns about the technical difficulties of in-office arthroscopy, the potential for patient discomfort, and the cumbersomeness of in-office arthroscopy units. However, significant advances have been made in the resolution capability of small-bore needle arthroscopy, resulting in much less painful procedures. Additionally, the early hardware designs, which mimicked operating room setups using towers, fluid irrigation systems, and larger arthroscopes, have been replaced with small-needle arthroscopes that use syringes for irrigation and tablet computers for visualization.
The mi-eye+TM technology (Trice Medical) is a small-bore needle unit for in-office arthroscopy with digital optics that does not need an irrigation tower. We conducted a pilot study of the sensitivity and specificity of the mi-eye+TM unit in comparison with MRI, using surgical arthroscopy as a gold-standard reference. We hypothesized that the mi-eye+TM needle arthroscope, which can be used in an office setting, would be equivalent to the standard of care (MRI) for the diagnosis of intra-articular pathology of the knee.