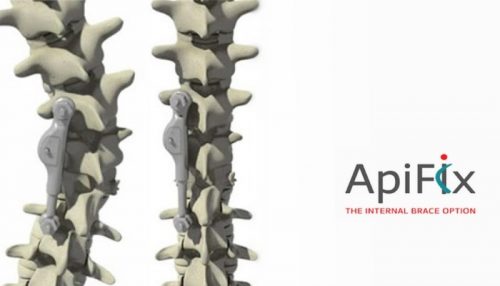
Misgav, Israel – 2 January 2018 –ApiFix Ltd. (“ApiFix”), a portfolio company of The TrendlinesGroup Ltd. (“Trendlines”) (SGX: 42T; OTCQX: TRNLY),announced the appointment of Ed Roschak as Chairman of the Board of ApiFix and Paul Mraz as President and CEO of ApiFix. ApiFix provides minimally invasive non-fusion solutions for the treatment of spinal scoliosis.
Mr. Roschak served as President and Chief Executive Officer of Ellipse Technologies, Inc. from 2011 until 2016, when it was acquired by NuVasive, Inc. for US$410 million. Ellipse Technologies developed a magnetic growth rod technology for treating early onset scoliosis of the spine and for limb lengthening procedures.
Mr. Mraz brings 25+ years of spine and orthopedic medical device experience to ApiFix, having worked as CEO and in other senior positions in U.S.- and Israel-based companies. Mr. Mraz’s experience includes executive management (14 years as CEO), corporate strategy, product development and marketing, sales management, and business development – all on a global basis. Previously, Mr. Mraz served as managing partner of business strategy and leadership consulting firm OnPoint Advisors, which he founded in 2013. From 2006 to 2013, Mr. Mraz served as president and CEO of medical device and biologics company Cerapedics, Inc., which developed and is now commercializing biologic bone grafting products for the spine and orthopedic markets.
Todd Dollinger, CEO and Chairman of Trendlines and outgoing ApiFix chairman, commented, “We believe the deep experiences Ed and Paul bring make them ideal to take ApiFix to the next level and we are excited to welcome them on board. Trendlines is extremely proud of ApiFix’s achievements to date and we’re confident that the combined efforts of Ed and Paul will be instrumental in realizing ApiFix’s strategic focus on the U.S. and European markets as ApiFix finalizes its application for FDA clearance.” Dollinger cited Roschak’s substantial work in every aspect of building a scoliosis treatment company as key reasons ApiFix sought to bring him in as Chairman of the ApiFix Board. Similarly, Mraz has extensive leadership experience bringing innovative spine technologies to market for the benefit of patients and all stakeholders. With ApiFix’s U.S. market entry plans in process, Mraz will establish ApiFix’s U.S. headquarters in the Boston, Massachusetts USA area. R&D and manufacturing activities will remain in Israel under the leadership of ApiFix’s co-founder and CTO, Uri Arnin.
The ApiFix system is a disruptive platform technology with its less invasive, non-fusion scoliosis correction system implanted in a short procedure that maintains spine flexibility and requires only a brief recovery period. The ApiFix system has CE Mark certification and is available in Europe and Canada.
The ApiFix Board of Directors expresses its deep gratitude to Eran Feldhay, M.D., for his contributions as CEO. Dr. Feldhay’s leadership brought ApiFix through critical commercialization milestones positioning it well for this next evolution.
About The Trendlines Group Ltd.
Trendlines is an innovation commercialization company that invents, discovers, invests in, and incubates innovation-based medical and agricultural technologies to fulfill its mission to improve the human condition. As intensely hands-on investors, Trendlines is involved in all aspects of its portfolio companies from technology development to business building.Trendlines’ shares are traded on the Singapore Stock Exchange (SGX: 42T) and in the United States as an American Depositary Receipt (ADR) on the OTCQX (OTCQX: TRNLY).
About ApiFix Ltd.
ApiFix is a privately held, innovation-driven, medical device company developing a non-fusion platform technology for the less invasive surgical correction of adolescent idiopathic scoliosis (AIS) and early onset scoliosis (EOS). ApiFix is led by a team of highly-regarded spine surgeons and spine industry veterans.
*******
This press release has been prepared by The Trendlines Group Ltd. (the “Company”) and its contents have been reviewed by PrimePartners Corporate Finance Pte. Ltd. (the “Sponsor”) for compliance with the Singapore Exchange Securities Trading Limited (the “SGX-ST”) Listing Manual Section B: Rules of Catalist. The Sponsor has not verified the contents of this press release.
This press release has not been examined or approved by the SGX-ST. The Sponsor and the SGX-ST assume no responsibility for the contents of this press release, including the accuracy, completeness or correctness of any of the information, statements or opinions made or reports contained in this press release.
The contact person for the Sponsor is Ms. Gillian Goh, Director, Head of Continuing Sponsorship (Mailing Address: 16 Collyer Quay, #10-00 Income at Raffles, Singapore 049318 and E-mail:sponsorship@ppcf.com.sg)
Investor Contact Information: Israel
Judith Kleinman, Director Investor Relations & Corporate Communications The Trendlines Group judith@trendlines.com Tel: +972.72.260.7000
Singapore:
Reyna MEI Financial PR reyna@financialpr.com.sg Tel: +65.6438.2990