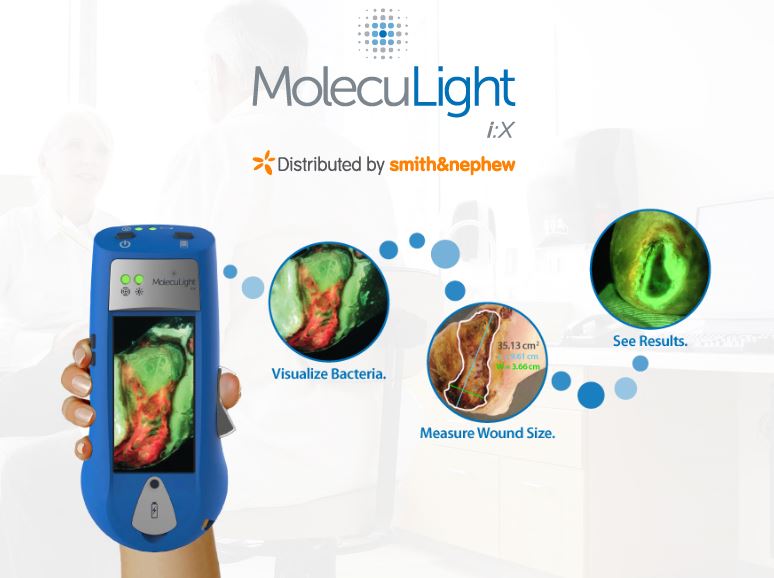
LONDON–(BUSINESS WIRE)–Smith & Nephew (LSE: SN, NYSE: SNN), the global medical technology business, today announces the European launch of MolecuLight i:XTM, the easy to use, handheld imaging device that instantly measures wound surface area and visualises the presence and distribution of potentially harmful bacteria in wounds.2,3
Currently wound assessments are made with the naked eye which can lack the accuracy required to most effectively guide clinical decision making.4 Using fluorescence, MolecuLight i:X quickly, safely, and easily visualises potentially harmful bacteria1,2,3 in wounds which may otherwise lack signs or symptoms of infection. It enhances a clinician’s ability to choose the right therapy, at the right time for their patient1,2 and can help to guide wound sampling and debridement,1,5,6 monitor wound progression,2,3 improve patient engagement4,5and simplify wound documentation.1
Clinical data from wound assessments demonstrates that incorporating the MolecuLight i:X into standard care facilitated more objective medical decision making and led to up to 9 times faster wound healing1 and 54% more accurate swabbing.7
“The MolecuLight i:X enables healthcare professionals to see what they have never been able to see before“ said Paolo Di Vincenzo, Smith & Nephew’s Senior Vice President Global Marketing, Wound. “We are proud to partner with MolecuLight Inc. and bring this innovative technology to our customers. It strongly complements our range of advanced wound care products and we believe is set to start a revolution in wound care clinical practice.”
“For the first time clinicians can accurately sample a wound in situ to determine if bacteria are present as well as more effectively debride a wound under fluorescence visualisation. These are fundamental areas of everyday wound care that have remained suboptimal for too long, until now,” says Dr. Ralph DaCosta, Founder, Chief Scientific Officer and Director, MolecuLight Inc.
An estimated 2 million people are living with a chronic wound across Europe and an estimated 16% of all chronic wounds remain unresolved after a year or longer.9,10,11 Ensuring wounds are appropriately diagnosed and treated is a priority for healthcare providers across Europe, reducing cost and improving outcomes for patients.
“Not only has the MolecuLight i:X transformed my department’s clinical decision making in terms of targeting sampling and debridement and improving antimicrobial stewardship, but the benefit to patients has also been exciting to see,” says Rosemary Hill*, Canadian Wound Ostomy Continence Nurse Clinician, Lions Gate Hospital, Vancouver. “By being able to engage patients in their wound healing progress, and by showing them the real-time images, we can reduce anxiety, and provide reassurance regarding the diminishing burden of bacteria.”
The MolecuLight i:X Imaging Device is approved by Health Canada (Medical License #95784) and has CE Marking (Certificate # G1160292355002) for sale in the European Union. The MolecuLight i:X Imaging Device is not available in the US.
Full press pack is available at: http://www.smith-nephew.com/news-and-media/media-releases/news/european-launch-of-handheld-imaging-technology-heralds-new-era-of-evidence-based-decision-making
About Smith & Nephew
Smith & Nephew is a global medical technology business dedicated to helping healthcare professionals improve people’s lives. With leadership positions in Orthopaedic Reconstruction, Advanced Wound Management, Sports Medicine and Trauma & Extremities, Smith & Nephew has more than 15,000 employees and a presence in more than 100 countries. Annual sales in 2016 were almost $4.7 billion. Smith & Nephew is a member of the FTSE100 (LSE:SN, NYSE:SNN).
For more information about Smith & Nephew, please visit our website www.smith-nephew.com, follow @SmithNephewplc on Twitter or visit SmithNephewplc on Facebook.com.
To learn more about what we do to help reduce wound infection, please visit www.closertozero.com
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as “aim”, “plan”, “intend”, “anticipate”, “well-placed”, “believe”, “estimate”, “expect”, “target”, “consider” and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith & Nephew, these factors include: economic and financial conditions in the markets we serve, especially those affecting health care providers, payers and customers; price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers; competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith & Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith & Nephew’s most recent annual report on Form 20-F, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith & Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith & Nephew are qualified by this caution. Smith & Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith & Nephew’s expectations.
◊ Trademark of Smith & Nephew. Certain marks registered US Patent and Trademark Office.
About MolecuLight Inc.
MolecuLight Inc. is a privately owned, Canadian medical imaging company delivering real-time fluorescence image-guidance solutions that provide clinicians with new information about wound bacterial burden and wound surface area to assist clinicians in making improved diagnostic and treatment decisions.
The MolecuLight i:X is manufactured by MolecuLight® Inc.
MaRS Centre, South Tower 101 College St., Suite 200 Toronto, ON M5G 1L7 Canada
T +1 647.362.4684 F +1 647.362.4730
www.moleculight.com
® Registered trademark acknowledged
[tm] All trademarks acknowledged
MolecuLight i:X and Look to Heal are registered trademarks of MolecuLight Inc in Canada, the US and the UK. MolecuLight DarkDrape is a registered trademark of MolecuLight® Inc in Canada. Other jurisdictions pending
The MolecuLight i:X is distributed by Smith & Nephew
Wound Management
Smith & Nephew Medical Ltd, 101 Hessle Road, Hull HU3 2BN, UK
T +44 (0) 1482 225181 F +44 (0) 1482 328326
www.smith-nephew.com
All Trademarks acknowledged
©October 2017 Smith & Nephew
AWM-AWD-11878
References:
1. DaCosta RS et al. Point-of-care autofluorescence imaging for real-time sampling and treatment guidance of bioburden in chronic wounds: first-in-human results. PLoS One. 2015 Mar 19;10(3).
2. MolecuLight Inc. PN 1189 MolecuLight i:X User Manual. 2016.
3. MolecuLight Inc. Case Study 0051 Track Wound Size and Bacterial Presence with the MolecuLight i:X. 2016.
4. Hoeflok J et al. Pilot clinical evaluation of surgical site infections with a novel handheld fluorescence imaging device. Proceedings of the Annual Military Health System Research Symposium (MHSRS); 2014 Aug 18- 21; Fort Lauderdale, FL.
5. Raizman R. Point-of-care fluorescence imaging device guides care and patient education in obese patients with surgical site infections. Presented at: CAWC 2016. Proceedings of the Annual Canadian Association of Wound Care Conference (CAWC); 2016 Nov 3-6, Niagara Falls, ON.
6. Raizman R. Fluorescence imaging positively predicts bacterial presence and guides wound cleaning and patient education in a series of pilonidal sinus patients. Proceedings of the Annual Wounds UK Conference; 2016 Nov 14-16; Harrogate, UK.
7. Ottolino-Perry K et al. Improved detection of wound bacteria using fluorescence image guided wound sampling in diabetic foot ulcers. Int Wound J. 2017 Feb 28. doi: 10.1111/iwj.12717.
8. Posnett J et al. J. Wound Care (April 2009), The Resource Impact of Wounds on Health-care Providers in Europe, vol.18 (4).
9. Lindholm C and Searle R. Wound management for the 21st century: combining effectiveness and efficiency. Int Wound J. 2016 Jul;13 Suppl 2:5-15.
10. Siddiqui AR and Bernstein JM. Chronic wound infection: facts and controversies. Clin Dermatol. 2010;28:519–26.
11. Vowden P. Hard-to-heal wounds made easy. Wounds International, Schofield Healthcare Media Ltd: Norwich, UK, 2011;2. URL: http:// www.woundsinternational.com.
* Rosemary Hill is a paid consultant of Smith & Nephew or MolecuLight Inc.