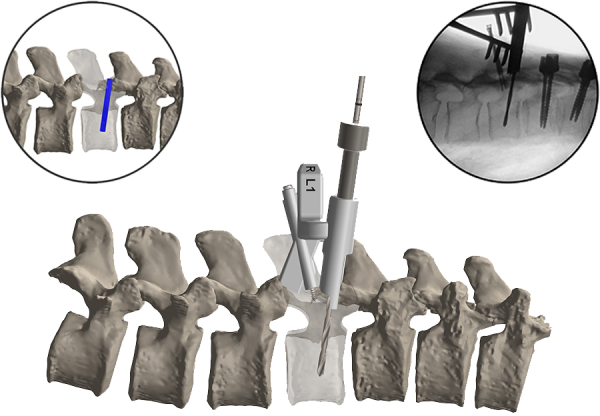
CAESAREA, Israel–(BUSINESS WIRE)– Mazor Robotics Ltd. (MZOR), a pioneer and a leader in the field of surgical guidance systems, today announced that it has entered the next phase of its strategic partnership with Medtronic earlier than planned and their existing agreements have been amended accordingly. The agreements provide for the conversion of the commercial relationship between the parties, with Medtronic assuming exclusive worldwide distribution of the Mazor X system, and Medtronic making a $40 million third tranche investment in Mazor. These developments are a result of the early achievement of certain sales and marketing milestones by both companies, as well as higher than expected global market acceptance and demand for the Mazor X system. Medtronic and Mazor originally entered into a strategic agreement in May 2016.
Key Highlights Include:
- Medtronic assumes exclusive global spine market commercial responsibility for the Mazor X Surgical Assurance Platform and its accessories.
- The implementation of annual minimums for purchase of Mazor X systems by Medtronic with a cumulative potential of hundreds of Mazor X systems over a four and a half-year period.
- Approximately 30 members of the current Mazor sales organization are expected to join Medtronic to assure continuation of the current momentum.
- Co-development of future products for the spine market that combine Mazor Robotics’ core expertise in surgical planning and precision-guided surgical systems with Medtronic’s navigation capabilities and implant systems. The first results of this combined and synergistic effort are expected to be demonstrated this fall.
- Mazor will continue to provide service to the global installed base of the Mazor X.
- A shared economic incentive to continue developing products and services that maintain innovation leadership and utilization of Mazor Robotics clinical solutions.
- Medtronic will invest $40 million in Mazor Robotics’ American Depository Shares (ADS) at a price of $38.46 per ADS, which represents the weighted average of the closing price of Mazor’s ADS on Nasdaq over the past 20 trading days. This third tranche of investment in Mazor by Medtronic will bring Medtronic’s total investment in Mazor to $72 million, representing approximately 11.9% of the outstanding shares post investment and 10.6% of the fully diluted shares outstanding post investment. Mazor will also issue to Medtronic warrants to purchase an additional 1.21 million Mazor ADSs at an exercise price of $44.23 per ADS. The exercise price represents a 15% premium over the per share price for the $40 million equity investment. Medtronic has the right to exercise the warrants immediately in whole or in part, for cash, and they expire after 18 months. Assuming the full exercise of the warrants, Medtronic’s investment in Mazor will reach $125 million and its ownership could increase to 4.2 million ADSs, or 14.2%, based on the current number of ADSs outstanding on a fully diluted basis. Closing of the $40 million equity investment is expected to take place on or around September 12, 2017.
“Medtronic is our valued strategic partner and together we have achieved the desired outcome for Phase I well ahead of our original plan,” commented Ori Hadomi, Chief Executive Officer. “I believe that the move to this next phase reinforces our significant leadership position in the growing market for surgical guidance systems for spine procedures. Our strategic partnership will allow hospitals in new markets around the world to have access to the Mazor X and gain the clinical benefits that this technology offers.
“The strategic partnership between Mazor and Medtronic has already resulted in 59 Mazor X system orders since the October 2016 launch and reflects an accelerated sales cycle due to customers’ eagerness to adopt our solutions for the spine market,” added Mr. Hadomi. “Now, as commercial responsibility for the Mazor X in the spine market shifts to Medtronic, the annual minimums for sale of Mazor X systems agreed to by the two companies are expected to drive substantial improvement in Mazor’s financial results during the next several years. Together we will be able to further advance our robust jointly-developed product pipeline for the spine market, to make a difference for patients while Mazor also pursues new opportunities to apply our innovative technologies to other medical needs.”
“Moving to the next phase of our strategic partnership demonstrates our shared passion for transforming how spine surgery is done,” said Doug King, senior vice president & president of the Medtronic Spine division, which is part of Medtronic’s Restorative Therapies Group. “Mazor Robotics’ technology and Medtronic’s navigation capabilities and implant systems provide spine surgeons with complete procedural solutions that advance the standard of care and will help surgeons maximize predictability and efficiency.”
Mazor will continue to manufacture and recognize revenues for Mazor X system sales, disposable kits and service fees all of which will be sold at contractual pricing agreed with Medtronic. The contracted pricing is at a lower rate than Mazor realized through its direct sales channel. In addition, Mazor will be entitled to certain synergy fees associated with the use of Medtronic implants in Mazor Robotics’ installed base. Moving from direct sales to a strategic distribution model is expected to immediately reduce Mazor’s annual operating expenses by approximately $13 million. Trailing 12-month operating expenses for Mazor totaled $52.7 million.
The proceeds from the investment will further strengthen Mazor’s balance sheet and provide the resources to continue to collaborate with Medtronic to develop innovative solutions for the spine market, as well as develop innovative solutions for other potential markets.
Mazor will continue to independently develop and market globally the Renaissance Surgical Guidance System, which was first launched in 2011. Efforts for Renaissance will be focused on certain market segments for which the Renaissance provides significant customer added value.
CONFERENCE CALL INFORMATION
The Company will host a conference call to discuss today’s announcement at 8:30 AM EDT (3:30 PM IDT). Investors within the United States interested in participating are invited to call 1-800-289-0498. Participants in Israel can use the toll-free dial-in number 1-80-925-8350. All other international participants can use the dial-in number 1-719-325-4818. For all callers, refer to Conference ID 1217133.
A replay of the event will be available for two weeks following the conclusion of the call. To access the replay, callers in the United States can call 1-866-375-1919 and reference the Replay Access Code: 1217133. All international callers can dial 1-719-457-0820, using the same Replay Access Code. To access the webcast, please visit www.mazorrobotics.com and select ‘Investor Relations.’
About Mazor
Mazor Robotics believes in healing through innovation by developing and introducing revolutionary technologies and products aimed at redefining the gold standard of quality care. Mazor Robotics Guidance System enables surgeons to conduct spine and brain procedures in an accurate and secure manner. For more information, please visit www.MazorRobotics.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other securities laws. Any statements in this release about future expectations, plans or prospects for the Company, including without limitation, statements containing the words “believes,” “anticipates,” “plans,” “expects,” “will” and similar expressions are forward-looking statements. These statements are only predictions based on Mazor’s current expectations and projections about future events. There are important factors that could cause Mazor’s actual results, level of activity, performance or achievements to differ materially from the results, level of activity, performance or achievements expressed or implied by the forward-looking statements. For example, Mazor is using forward-looking statements in this press release when it discusses the outcome of the Phase II agreements with Medtronic, when it states that the results of combined development efforts are expected to be launched this fall, when it states that sales minimums are expected to drive substantial improvement in Mazor’s financial results during the next several years, when it discusses that the strategic partnership will allow hospitals in new markets around the world to have access to the Mazor X and gain the clinical benefits that this technology offers, when benefits spine surgeons will get from the combination of Mazor’s technology and Medtronic’s navigation capabilities and implant systems are discussed, when it discusses advancing its robust, jointly-developed product pipeline for the spine market while pursuing exclusive efforts to apply its innovation to other medical needs, when it discusses that moving from direct sales to a strategic distribution model is expected to immediately reduce its annual operating expenses, when it discusses continuing to independently develop and market globally the Renaissance Surgical Guidance System and where it will focus its efforts with regard to this system, and when it discusses the timing of the closing of the Medtronic purchase of ADSs and the potential exercise of warrants as well as the use of proceeds from the sale of such securities. Those factors include, but are not limited to, the impact of general economic conditions, competitive products, product demand and market acceptance risks, reliance on key strategic alliances, fluctuations in operating results, and other factors indicated in Mazor’s filings with the Securities and Exchange Commission (SEC) including those discussed under the heading “Risk Factors” in Mazor’s annual report on Form 20-F filed with the SEC on May 1, 2017 and in subsequent filings with the SEC. For more details, refer to Mazor’s SEC filings. Mazor undertakes no obligation to update forward-looking statements to reflect subsequent occurring events or circumstances, or to changes in our expectations, except as may be required by law.
View source version on businesswire.com: http://www.businesswire.com/news/home/20170829006252/en/
EVC Group
Michael Polyviou, 212-850-6020
mpolyviou@evcgroup.com
or
Doug Sherk, 646-445-4800
dsherk@evcgroup.com
Source: Mazor Robotics Ltd.