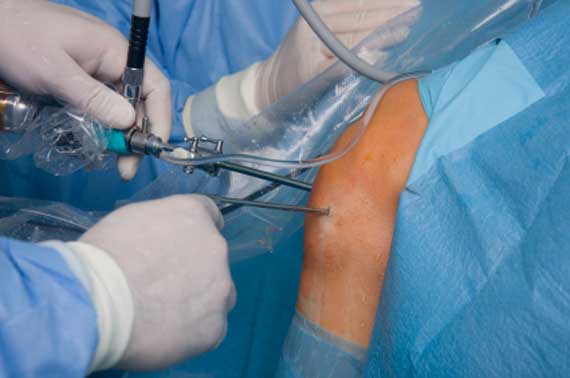
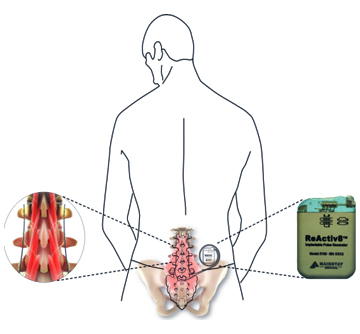
Mainstay Medical International plc (“Mainstay” or the “Company”, Euronext Paris: MSTY.PA and ESM of the Irish Stock Exchange: MSTY.IE), a medical device company focused on bringing to market ReActiv8®, an implantable restorative neurostimulation system to treat disabling Chronic Low Back Pain (“CLBP”), announces that over half the required number of implants in the ReActiv8-B Clinical Trial have been performed.
The trial is intended to gather data in support of an application for pre-market approval (PMA) from the US Food and Drug Administration (FDA), a key step towards commercialization of ReActiv8 in the US. Information about the trial can be found at https://clinicaltrials.gov/show/NCT02577354.
69 subjects have been implanted with ReActiv8 in the trial. The trial design requires 128 subjects in the pivotal cohort to reach the 120-day endpoint before data are made available. An “interim look” for sample size re-estimation is planned when half the implanted subjects have data from the 120-day visit. The enrolment rate has been accelerating as the number of active sites has increased during 2017.
ReActiv8 is designed to electrically stimulate the nerves responsible for contracting muscles which stabilize the lumbar spine. Activation of these muscles to restore functional stability has been shown to facilitate recovery from CLBP. Mainstay received CE Marking for ReActiv8 in May 2016 based on positive results from the ReActiv8-A clinical trial which demonstrated a clinically important, statistically significant and lasting improvement in pain, disability and quality of life in people with disabling CLBP and few other treatment options.Mainstay has begun commercialization in Europe, focusing initially on Germany, where the Company aims to drive adoption of ReActiv8 in a select number of high volume multi-disciplinary spine care centers. More recently, commercialization has begun in Ireland, Mainstay’s home market.
Peter Crosby, CEO of Mainstay, commented: “The ReActiv8-B Clinical Trial is advancing well, and, based on our experience to date, we anticipate completing enrolment around the end of this year, with results available in 2018. The ReActiv8-B trial is a key step towards commercialization in the US, our most significant target market, and we are pleased with the progress.
“Our initial commercialization of ReActiv8 in Europe is well underway. Our strategy is to work with key reference centers in Germany, and then build on that experience and data from the ReActiv8-B Trial to expand commercialization to additional centers and other countries.”
ReActiv8-B Clinical Trial
The ReActiv8-B Clinical Trial is an international, multi-center, prospective randomized sham controlled triple blinded trial with one-way crossover, conducted under an Investigational Device Exemption (IDE). The statistical design of the Clinical Trial requires data from the pivotal cohort of 128 randomized subjects at the 120-day primary outcome assessment visit. Total number of subjects implanted will also include some enrolled and implanted as part of the surgical roll-in phase, in addition to subjects in the pivotal cohort. The Trial is designed with an “interim look” for sample size re-estimation when primary outcome data are available from half the subjects in the pivotal cohort, and if necessary the number of subjects in the pivotal cohort may be increased to achieve the targeted statistical significance. The interim analysis will be performed by a third-party independent statistician under the direction of the Data Monitoring Committee (DMC), and the interim results, other than a DMC recommendation regarding the findings, will remain blinded to the Company, study subjects, investigators and Clinical Trial sites.
The primary efficacy endpoint of the ReActiv8-B Clinical Trial is a comparison of responder rates between the treatment and control arms. The Clinical Trial will be considered a success if there is a statistically significant difference in responder rates between the treatment and control arms. The Clinical Trial, if successful, will provide what is referred to as Level 1A Evidence of efficacy of ReActiv8, which may be used to support applications for favorable reimbursement in the USA. Evidence from the ReActiv8-B Trial will also be used to support market development activities worldwide.
About Mainstay
Mainstay is a medical device company focused on bringing to market an innovative implantable restorative neurostimulation system, ReActiv8®, for people with disabling Chronic Low Back Pain (CLBP). The Company is headquartered in Dublin, Ireland. It has subsidiaries operating in Ireland, the United States, Australia and Germany, and its ordinary shares are admitted to trading on Euronext Paris (MSTY.PA) and the ESM of the Irish Stock Exchange (MSTY.IE).
About the ReActiv8-B Clinical Trial
The ReActiv8-B Clinical Trial is an international, multi-center, prospective randomized sham controlled blinded trial with one-way crossover conducted under an Investigational Device Exemption (IDE). The ReActiv8-B Clinical Trial is designed to generate data to form part of the Pre-Market Approval Application (PMAA) of ReActiv8 to the FDA. Further details can be found at https://clinicaltrials.gov/show/NCT02577354
About Chronic Low Back Pain
One of the recognized root causes of CLBP is impaired control by the nervous system of the muscles that dynamically stabilise the spine in the low back, and an unstable spine can lead to back pain. ReActiv8 is designed to electrically stimulate the nerves responsible for contracting these muscles and thereby help to restore muscle control and improve dynamic spine stability, allowing the body to recover from CLBP.
People with CLBP usually have a greatly reduced quality of life and score significantly higher on scales for pain, disability, depression, anxiety and sleep disorders. Their pain and disability can persist despite the best available medical treatments, and only a small percentage of cases result from an identified pathological condition or anatomical defect that may be correctable with spine surgery. Their ability to work or be productive is seriously affected by the condition and the resulting days lost from work, disability benefits and health resource utilization put a significant burden on individuals, families, communities, industry and governments.
Further information can be found at www.mainstay-medical.com
CAUTION – in the United States, ReActiv8 is limited by federal law to investigational use only.
PR and IR Enquiries:
Consilium Strategic Communications (international strategic communications – business and trade media)
Chris Gardner, Mary-Jane Elliott, Jessica Hodgson, Hendrik Thys
Tel: +44 203 709 5700 / +44 7921 697 654
Email: mainstaymedical@consilium-comms.com
or
FTI Consulting (for Ireland)
Jonathan Neilan
Tel: +353 1 765 0886
Email: jonathan.neilan@fticonsulting.com
or
NewCap (for France)
Louis-Victor Delouvrier
Tel: +: +33 1 44 71 98 53
Email: lvdelouvrier@newcap.fr
or
AndreasBohne.Com/Kötting Consulting (for Germany)
Andreas Bohne
Tel : +49 2102 1485368
Email : abo@andreasbohne.com
Wilhelm Kötting
Tel: +49 69 75913293
Email: wkotting@gmail.com
or
Investor Relations:
LifeSci Advisors, LLC
Brian Ritchie
Tel: +1 (212) 915-2578
Email: britchie@lifesciadvisors.com
or
ESM Advisers:
Davy
Fergal Meegan or Barry Murphy
Tel: +353 1 679 6363
Email: fergal.meegan@davy.ie or barry.murphy2@davy.ie
Forward looking statements
This announcement includes statements that are, or may be deemed to be, forward looking statements. These forward looking statements can be identified by the use of forward looking terminology, including the terms “anticipates”, “believes”, “estimates”, “expects”, “intends”, “may”, “plans”, “projects”, “should”, “will”, or “explore” or, in each case, their negative or other variations or comparable terminology, or by discussions of strategy, plans, objectives, goals, future events or intentions. These forward looking statements include all matters that are not historical facts. They appear throughout this announcement and include, but are not limited to, statements regarding the Company’s intentions, beliefs or current expectations concerning, among other things, the Company’s results of operations, financial position, prospects, financing strategies, expectations for product design and development, regulatory applications and approvals, reimbursement arrangements, costs of sales and market penetration.
By their nature, forward looking statements involve risk and uncertainty because they relate to future events and circumstances. Forward looking statements are not guarantees of future performance and the actual results of the Company’s operations, and the development of its main product, the markets and the industry in which the Company operates, may differ materially from those described in, or suggested by, the forward looking statements contained in this announcement. In addition, even if the Company’s results of operations, financial position and growth, and the development of its main product and the markets and the industry in which the Company operates, are consistent with the forward looking statements contained in this announcement, those results or developments may not be indicative of results or developments in subsequent periods. A number of factors could cause results and developments of the Company to differ materially from those expressed or implied by the forward looking statements including, without limitation, the successful launch and commercialisation of ReActiv8®, the progress and success of the ReActiv8-B Clinical Trial, general economic and business conditions, the global medical device market conditions, industry trends, competition, changes in law or regulation, changes in taxation regimes, the availability and cost of capital, the time required to commence and complete clinical trials, the time and process required to obtain regulatory approvals, currency fluctuations, changes in its business strategy, political and economic uncertainty. The forward-looking statements herein speak only at the date of this announcement.
Short Name: Mainstay Medical
Category Code: STR
Sequence Number: 600858
Time of Receipt (offset from UTC): 20170802T174402+0100