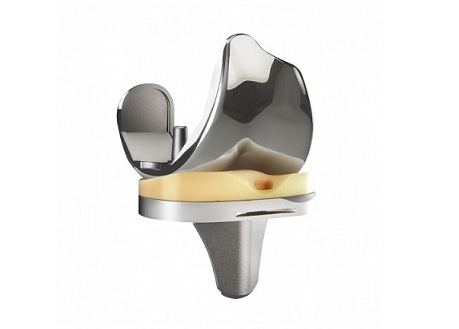
AUSTIN, Texas–(BUSINESS WIRE)–DJO (“DJO” or the “Company”), a leading provider of medical technologies designed to get and keep people moving, released a new clinical study to show that EMPOWR 3D Knee technology reduces patient dissatisfaction, and improves function and activity levels. Based on a multitude of studies on the biomechanics and kinematics of the knee joint, and more than 10 years of clinical heritage with DJO’s 3D Knee™, the EMPOWR 3D Knee® is the industry’s first “dual-pivot” technology, a breakthrough concept that is designed to better replicate the natural motion and feeling of the healthy knee.
With nearly 700,000 procedures performed in the U.S. each year, total knee replacement surgery (TKR) is one of the most widely accepted and effective treatments for relieving degenerative osteoarthritis pain and suffering. Unfortunately, nearly 20 percent of all patients – approximately 140,000 Americans each year1 – report they are not satisfied after their procedure, often claiming that their knee replacement “does not feel normal.”
EMPOWR 3D Knee® is one of the most functionally accurate prostheses ever created.1 EMPOWR 3D Knee®more closely recreates the natural dual-pivot motion pattern of the healthy knee throughout the entire range of motion, from early to deep flexion, creating a more natural feeling knee for the patient.1
Short term clinical results recently presented at the International Congress for Joint Reconstruction Hip and Knee Course in June 2018, demonstrate that the unique dual-pivot design of the EMPOWR 3D Knee® may offer far more natural motion for total knee arthroplasty (TKA) patients by more closely recreating the kinematics of a healthy human knee.2
The most significant clinical benefits of the EMPOWR 3D Knee® identified in the study include2:
- Patient dissatisfaction is reduced by 50% compared to a competitive implant
- Patient’s “knee feels normal” ~25% more often than with a competitive implant
- Patients are over ~2X more active following surgery with the EMPOWR 3D Knee® versus a competitive knee implant
“Initial clinical results have been very encouraging for the EMPOWR 3D Knee®,” said presenter Dr. Michael Meneghini, MD, Associate Professor of Orthopedic Surgery at Indiana University School of Medicine. “Patients with the EMPOWR 3D dual-pivot knee implant are experiencing improved knee function and showing a greater likelihood of a normal feeling knee.”2
Dr. Scott Banks, PhD, Professor of Orthopedic and Joint Mechanics at the University of Florida, and Dr. Meneghini recently published an article in the journal Techniques in Orthopedics comparing the EMPOWR 3D Knee® implant to traditional knee implant designs. They concluded that contemporary dual-pivot design and kinematics of the EMPOWR 3D Knee® may contribute to improved function and satisfaction after TKR.1
“Clinical studies over the past decade have shown that healthy knees have a much more complex front-to-back and side-to-side motion than previously believed,” said Banks. “As a result, we now better understand why traditional knee arthroplasty designs have failed to accurately replicate normal knee activity and results in many patients feeling dissatisfied with their knee implant.”
“EMPOWR 3D Knee® patients tell us, ‘this knee just feels like my own,’ and that’s the whole point,” said Jeffery A. McCaulley, Global President of DJO Surgical®. “EMPOWR 3D Knee® goes beyond clinical effectiveness to deliver one of the most functionally accurate knee implants based on our novel dual-pivot design that helps to improve patient outcomes and patient satisfaction.”
For more information on the EMPOWR 3D Knee®, please visit: www.djoglobal.com/empowr.
1. Banks, Scott A., and Robert M. Meneghini. “Achieving More Natural Motion, Stability, and Function With a Dual-Pivot ACL-substituting Total Knee Arthroplasty Design.” Techniques in Orthopaedics 33, no. 1 (2018): 48-51.
2. “Replicating Native Knee Dual-Pivot Kinematics Improves Outcomes After Total Knee Arthroplasty” R. Michael Meneghini MD – ICJR South Presentation June 2018.
R. Michael Meneghini, MD is a consultant for DJO Global®.
Scott A. Banks, MD is a consultant for DJO Global®.
About DJO®
DJO® is a leading global developer, manufacturer and distributor of high-quality medical devices that provide solutions for musculoskeletal health, vascular health and pain management. The Company’s products address the continuum of patient care from injury prevention to rehabilitation after surgery, injury or from degenerative disease, enabling people to regain or maintain their natural motion. Its products are used by orthopedic specialists, spine surgeons, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers and other healthcare professionals.
In addition, many of the Company’s medical devices and related accessories are used by athletes and patients for injury prevention and at-home physical therapy treatment. The Company’s product lines include rigid and soft orthopedic bracing, hot and cold therapy, bone growth stimulators, vascular therapy systems and compression garments, therapeutic shoes and inserts, electrical stimulators used for pain management and physical therapy products. The Company’s surgical division offers a comprehensive suite of reconstructive joint products for the hip, knee and shoulder. DJO Global’s products are marketed under a portfolio of brands including Aircast®, Chattanooga®, CMF™, Compex®, DonJoy®, ProCare®, DJO Surgical®, Dr. Comfort® and Exos®. For additional information on the Company, please visit www.DJOGlobal.com.