September 20, 2018
ALLENDALE, N.J.–(BUSINESS WIRE)–Stryker’s Spine division will feature its family of 3D-printed Tritanium interbody fusion cages and highlight its Tritanium In-Growth Technology1 in an augmented reality experience at the North American Spine Society Annual Meeting, Sept. 26–29, 2018, in Los Angeles (booth No. 1401).
The augmented reality “tour” combines objects in the real world with computer-augmented 3D animations. Using this augmented reality storyline, as well as a series of hands-on activities and scientific animations, the experience at the Stryker booth is designed to enhance understanding of the story behind Tritanium In-Growth Technology, as well as AMagine™, Stryker’s proprietary additive manufacturing (or 3D-printing) process, which assists in the creation of highly porous implants with a structure that is designed to mimic bone.
“Our augmented reality tour will demonstrate Tritanium’s characteristics, with the goal of helping surgeons better understand the differences of materials on the market,” said Michael Carter, vice president and general manager of Stryker’s Spine division. “Tritanium continues to receive terrific feedback as more surgeons become believers in the technology, validating our commitment to providing advanced and innovative products for our surgeon customers and their patients.”
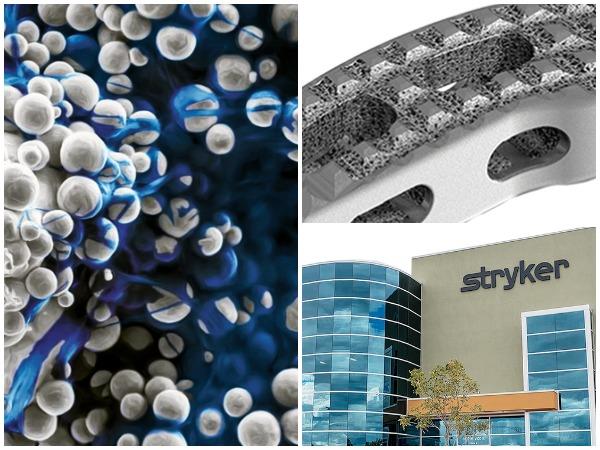
Tritanium cages are inspired by the microstructure of cancellous bone1 and enabled by AMagine, Stryker’s proprietary approach to implant creation using additive manufacturing. The AMagine process allows the company to tackle previously difficult or impossible design complexities and address unmet surgeon needs with unique material characteristics. For example, Tritanium may be able to wick and retain fluid inside its porosity, compared to traditional titanium.1,2 Additionally, the unique porous structure of Tritanium Technology was designed to create a favorable environment for bone cells to attach and proliferate.3,4*
During the NASS conference, Stryker will also:
- Feature results of a recent comparative animal study, which found significant differences in biomechanical and histologic performance among various interbody cage materials. The results showed that Tritanium cages achieved statistically significant increases in bone in-growth.4
- Present a Solution Showcase titled “A Clinical Update on Tritanium—A Novel, Highly Porous Material Designed for Bone In-Growth and Biological Fixation,” by Scott Parker, M.D., assistant professor of neurological surgery, Vanderbilt University Medical Center, on Sept. 26, 2018, from 12–12:20 p.m. PT.
- Preview its new Tritanium TL Curved Posterior Lumbar Cage, which received 510(k) clearance from the U.S. Food and Drug Administration in January 2018. Click here for the indications for use.
About Stryker
Stryker is one of the world’s leading medical technology companies and, together with its customers, is driven to make healthcare better. The company offers innovative products and services in Orthopaedics, Medical and Surgical, and Neurotechnology and Spine that help improve patient and hospital outcomes. More information is available at www.stryker.com and www.builttofuse.com. Follow Stryker’s Spine division on Twitter @stryker_spine.
References
| 1. | Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26, 5475-5491. | |
| 2. | RD0000053710: Tritanium cell infiltration and attachment experiment. | |
| *No correlation to human clinical outcomes has been demonstrated or established. | ||
| 3. | RD0000050927: Tritanium material capillary evaluation. | |
| 4. | McGilvray KC, Easley J, Seim HB, et al. Bony ingrowth potential of 3D-printed porous titanium alloy: a direct comparison of interbody cage materials in an in vivo ovine lumbar fusion model. Spine J. 2018;18(7):1250-1260. |
CONTENT ID TRITA-PB-4
A surgeon must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that surgeons be trained in the use of any particular product before using it in surgery.
The information presented is intended to demonstrate the breadth of Stryker product offerings. A surgeon must always refer to the package insert, product label and/or instructions for use before using any Stryker product. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in your area.
Dr. Parker is a paid consultant of Stryker. His/her statements represent his/ her own opinions based on personal experience and are not necessarily those of Stryker. Individual results may vary.
Contacts
Stryker’s Spine Division
Jodie Morrow
Jodie.morrow@stryker.com
or
Sullivan & Associates
Andrea Sampson
asampson@sullivanpr.com, 714/374–6174