July 02, 2018
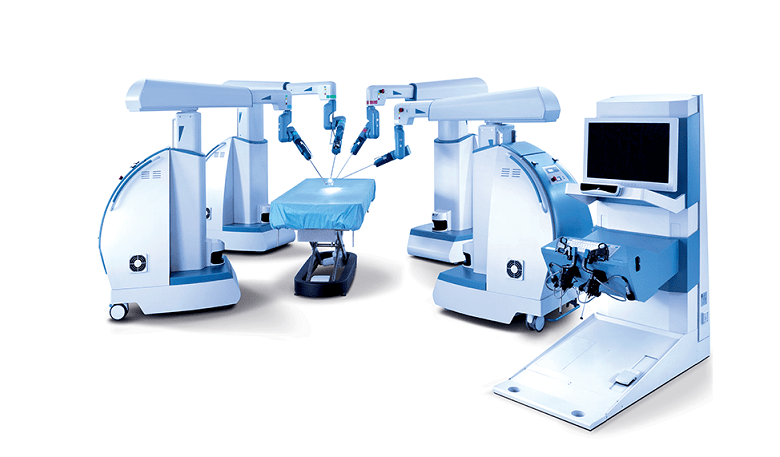
RESEARCH TRIANGLE PARK, N.C.–(BUSINESS WIRE)–TransEnterix, Inc. (NYSE American:TRXC), a medical device company that is digitizing the interface between surgeons and patients to improve minimally invasive surgery, today provided a corporate update, including the announcement of the sale of an additional Senhance system as well as preliminary unaudited revenue for the second quarter ended June 30, 2018.
“We had a strong second quarter as we continued to drive sales of Senhance globally while at the same time making significant progress towards our 2018 goals, including the expansion of Senhance’s indications for use and portfolio of instruments,” said Todd M. Pope, President and CEO at TransEnterix. “We look forward to continuing to build upon the momentum we developed during the first half of the year to drive the widespread adoption of Senhance.”
Second Quarter Senhance System Sales
In June of 2018, the Company sold a Senhance System to an end user hospital through a distributor in the Company’s EMEA (Europe, Middle East, and Africa) region. This sale represents the fourth system sale during the second quarter of 2018, three of which (two in the EMEA region, one in the U.S.) have been previously announced.
Preliminary Second Quarter Revenue
Preliminary unaudited second quarter revenue is expected to be in the range of $6.0 million to $6.3 million, up from $1.5 million in the second quarter of 2017.
Indication Expansion
On May 29, 2018, the Company received FDA 510(k) clearance for expanded indications of its Senhance System for laparoscopic inguinal hernia and laparoscopic cholecystectomy (gallbladder removal) surgery. There are approximately 760,000 inguinal hernia and 1.2 million laparoscopic cholecystectomy procedures performed annually in the U.S. With this clearance, Senhance System’s total addressable annual procedures in the U.S. has more than doubled to over three million.
Instrument Portfolio Expansion
On June 7, 2018, the Company announced that it had filed an FDA 510(k) submission for additional Senhance System instruments, including 3 millimeter diameter instruments.
Balance Sheet
On May 23, 2018, the Company entered into a loan and security agreement providing the company with up to $40.0 million in term loans. The initial tranche of the term loan, $20 million, was received at closing. The Company will be eligible to draw on the second tranche of $10 million upon achievement of certain Senhance System revenue-related milestones for its 2018 fiscal year, and a third tranche of $10 million upon achievement of designated trailing six months GAAP net revenue from Senhance sales. On the date of closing, the Company repaid all amounts owed under their previous loan provider.
Preliminary unaudited cash and cash equivalents as of June 30, 2018 was approximately $98 million.
About TransEnterix, Inc.
TransEnterix is a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery by addressing the clinical and economic challenges associated with current laparoscopic and robotic options in today’s value-based healthcare environment. The Company is focused on the commercialization of the Senhance™ Surgical System, which digitizes laparoscopic minimally invasive surgery. The system allows for robotic precision, haptic feedback, surgeon camera control via eye sensing and improved ergonomics while offering responsible economics. The Senhance Surgical System is available for sale in the US, the EU and select other countries. For more information, visit www.transenterix.com.
Forward-Looking Statements
This press release includes statements relating to the Senhance Surgical System and our current regulatory and commercialization plans for this product. These statements and other statements regarding our future plans and goals constitute “forward looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. Such statements are subject to risks and uncertainties that are often difficult to predict, are beyond our control and which may cause results to differ materially from expectations and include whether we are making significant progress towards our 2018 goals and whether the preliminary unaudited 2018 second quarter revenue will be in the range of $6.0 million to $6.3 million. For a discussion of the risks and uncertainties associated with TransEnterix’s business, please review our filings with the Securities and Exchange Commission (SEC), including our Annual Report on Form 10-K filed on March 8, 2018 and our other filings we make with the SEC. You are cautioned not to place undue reliance on these forward looking statements, which are based on our expectations as of the date of this press release and speak only as of the origination date of this press release. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.
Contacts
For TransEnterix, Inc.
Investors:
Mark Klausner, +1-443-213-0501
invest@transenterix.com
or
Media:
Joanna Rice, + 1-951-751-1858
joanna@greymattermarketing.com