SAN DIEGO, CA–(Marketwired – October 25, 2016) – NuVasive, Inc. (NASDAQ: NUVA), a leading medical device company focused on transforming spine surgery with minimally disruptive, procedurally-integrated solutions, today announced the introduction of new spine innovations at the North American Spine Society (NASS) 31st Annual Meeting held October 26-29, 2016 at the Boston Convention and Exhibition Center. NuVasive will showcase the Company’s expanded Integrated Global Alignment™ (iGA) platform, now supporting all spinal procedures, including cervical alignment. In addition, the Company will introduce proprietary image enhancement software that allows the surgical staff to significantly reduce exposure to surgical radiation in the operating room (OR), and directly addresses a known safety concern of surgeons and OR staff.
iGA Platform for Cervical
iGA is a proprietary, procedurally-integrated digital platform of specialized products designed to help surgeons achieve more precise spinal column alignment. The expanded platform makes NuVasive the first company to offer a solution to address spinal alignment for all spine procedures and provides surgeons an intuitive surgical workflow during pre-, intra- and post-operative that consists of software and hardware. NuVasive is committed to a global approach for assessing, preserving, and restoring spinal alignment in an effort to promote surgical efficiencies, lasting patient outcomes, and improved quality of life.
NuVasive’s differentiated technology helps surgeons:
- Calculate the patient’s cervical alignment and preoperatively plan prior to surgery,
- Correct and assess the cervical spine intraoperatively through integrated procedural solutions, and
- Confirm postoperatively to reconcile the preoperative plan and the intraoperative assessment.
Part of the expanded platform includes the launch of Bendini® OCT, the innovative computer-assisted, rod bending system, which now has the ability to bend spinal rods for occipito-cervico-thoracic (OTC) cases. Bendini OCT is used in combination with NuvaPlanning™ software, integrated into surgical workflow for pre-, intra- and post-operative planning and confirmation, and NuvaMap™ O.R., the industry’s first and only real-time intraoperative assessment tool to assess the correction achieved prior to finishing the case. Also, as part of the cervical iGA platform, the Company will be launching the CoRoent® Small Interlock™ Hyperlordotic system, the first anterior cervical interfixated implant designed to treat cervical alignment deformities by offering multiple lordotic options to address patient-specific correction.
“Based on the identified clinical need and scientific foundation of driving improved clinical outcomes through optimized global sagittal alignment, we have expanded our iGA platform to now address cervical alignment. Building on the tremendous success the Company has seen with iGA, we are proud to offer the only available solution to address spinal alignment for all spine procedures,” said Jason Hannon, NuVasive’s president and chief operating officer. “This cutting-edge surgical planning technology enables surgeons to more completely plan for and achieve total sagittal alignment, including for patients suffering from cervical conditions. It represents another step forward in our mission to transform spine surgery.”
Improving OR Safety with LessRay® Technology
Physicians can exceed their lifetime Occupational Radiation Limit within the first decade of their career.
As surgery trends increasingly towards minimally invasive surgery (MIS) due to patient benefits, surgeon familiarity and superior clinical and economical outcomes, radiation exposure to surgeons and OR staff may increase.
To directly address this potential barrier to widespread adoption of MIS, NuVasive acquired the LessRay software technology suite from a private company called SafeRay Spine. The LessRay software is designed to be integrated into current surgeon workflow and utilizes an algorithm to drive image registration and enable management of radiation exposure, while maintaining high-quality, intra-operative images on existing C-Arm workflow without loss of visual accuracy.
“NuVasive has a strong history of delivering disruptive spine technology. From XLIF to iGA, we continually outpace our peers when addressing unmet clinical and market requirements. Radiation in the OR is a known issue, one that plagues the surgeon, the staff and the patient,” said Mr. Hannon. “This proprietary technology is aimed at supporting the surgeon and their staff, transforming their environment to be safer and more productive, while directly addressing one of the leading barriers to adopt MIS without any disruption to their familiar workflow. We see workflow familiarity as a key requirement for broad and rapid adoption of any successful technology platform.
“In addition, the technology fits our portfolio of differentiated solutions as a foundational element of our imaging, navigation and surgical automation development strategy. Our unmatched spine platform includes industry-leading hardware, software and services, transforming spine surgery through reproducible clinical and economic outcomes,” added Mr. Hannon.
NuVasive NASS Participation Details
NuVasive will showcase and demo its new technologies at NuVasive Booth #1315 on the NASS exhibit floor.
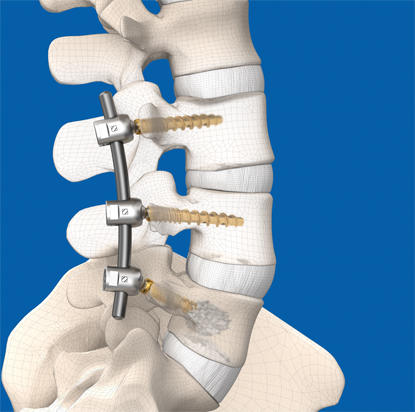
The Company will also feature the new BASE™ Interfixated Titanium system, a key addition to the iGA platform. BASE is designed to achieve fusion goals and spinal alignment objectives with precision, innovative functionality and a wide range of implant size and lordosis options. Titanium implants have a proven strength profile compared to that of other materials on the market. The new system offers versatile fixation options with a distinctive locking mechanism and anatomic implant design to help rebuild spinal foundation.
NuVasive’s participation at NASS includes a series of in-booth presentations and workshops. Visit the Company’s website for presentation details.
About NuVasive
NuVasive, Inc. (NASDAQ: NUVA) is a world leader in minimally invasive, procedurally-integrated spine solutions. From complex spinal deformity to degenerative spinal conditions, NuVasive is transforming spine surgery with innovative technologies designed to deliver reproducible and clinically proven surgical outcomes. NuVasive’s highly differentiated, procedurally-integrated solutions include access instruments, implantable hardware and software systems for surgical planning and reconciliation technology that centers on achieving the global alignment of the spine. With $811 million in revenues (2015), NuVasive has an approximate 2,200 person workforce in more than 40 countries around the world. For more information, please visit www.nuvasive.com.
Forward-Looking Statements
NuVasive cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that involve risks, uncertainties, assumptions and other factors which, if they do not materialize or prove correct, could cause NuVasive’s results to differ materially from historical results or those expressed or implied by such forward-looking statements. The potential risks and uncertainties which contribute to the uncertain nature of these statements include, among others, risks associated with acceptance of the Company’s surgical products and procedures by spine surgeons, development and acceptance of new products or product enhancements, clinical and statistical verification of the benefits achieved via the use of NuVasive’s products (including the iGA™ platform), the Company’s ability to effectually manage inventory as it continues to release new products, its ability to recruit and retain management and key personnel, and the other risks and uncertainties described in NuVasive’s news releases and periodic filings with the Securities and Exchange Commission.NuVasive’s public filings with the Securities and Exchange Commission are available at www.sec.gov. NuVasive assumes no obligation to update any forward-looking statement to reflect events or circumstances arising after the date on which it was made.
CONTACT INFORMATION
-
Investor Contact:
Suzanne Hatcher
NuVasive, Inc.
858-458-2240
Email contact
Media Contact:
Michael Farrington
NuVasive, Inc.
858-909-1940
Email contact