ATLANTA, GA–(Marketwired – Sep 21, 2016) – Medovex Corp. (NASDAQ: MDVX), a developer of medical technology products, today announced that the Company will exhibit during EuroSpine which is being held from October 5-7, 2016 in Berlin, Germany.
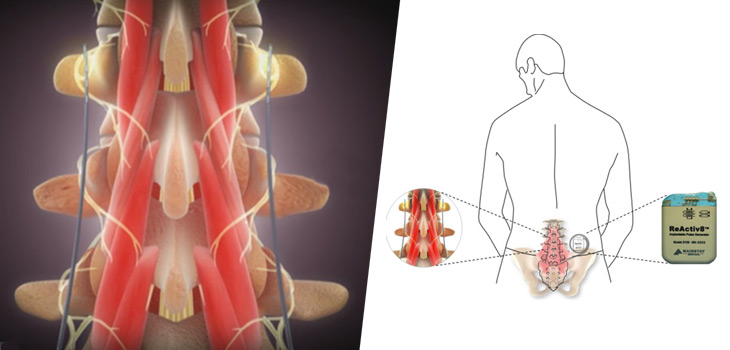
As part of the product exhibition, Medovex will highlight its innovative DenerveX™ System, a minimally invasive surgical (MIS) procedure pioneered by Medovex that was built around the combination of denervation and capsulectomy with reproducible results, “A potential paradigm shift in the treatment of Facet Joint pain.”
Medovex will exhibit at stand #B100 and will feature a number of work stations to demonstrate DenerveX™ treatment modality which uses Rotablation™ technology, high heat and rotational capsular tissue shaving, in a minimally invasive posterior capsulectomy procedure of the Facet Joint.
Medovex’s presence at EuroSpine is an extension of the Company’s continued investment in its strategic expansion throughout the EMEA market. EuroSpine is the union from the European Spine Society and the European Spinal Deformity Society. The Spine Society of Europe has experienced strong growth in membership, activities and participants at annual meetings, including participants from all over the world.
During EuroSpine 2016, Medovex also will announce the official opening of the Company’s European Distribution Center in Berlin.
The Center will serve as Medovex’s European distribution service headquarters and function as the commercial hub for all European distributors and end users, as well as other support functions. The international operations center in Atlanta, GA (USA) will remain the Company’s international operational headquarters.
Patrick Kullmann, President and COO for Medovex, stated, “Region specific events such as EuroSpine are important to our expansion efforts in Europe as we prepare for the future launch of the DenerveX System. The Berlin distribution service center will provide further infrastructure for Medovex to execute our future market development strategy across Western Europe and the entire EMEA region. Medovex is well-positioned to drive surgeon adoption of our new innovative DenerveX™ System across Europe.”
Manfred Sablowski, Senior Vice President of Global Sales & Marketing, added, “The opening of the European distribution service center is an important milestone for Medovex and a key inflection point for our Company’s strategy to grow our business in EMEA. In past months, we have additionally expanded our footprint to include all of Scandinavia and Israel. Our future goal is to increase our world-class EMEA distribution in other key countries. With the recent appointment of Juan Davila as Director Sales & Marketing for Latin America, having extensive experience in this market, we expect to continue to execute our go to market strategy.”
The DenerveX System consists of the DenerveX device, a single use medical device and the DenerveX Pro-40 Power Generator, both designed to be less invasive with faster recovery time than current surgical treatment options. It consists of two procedures combined into one device and is expected to provide for a longer lasting treatment solution while offering potential savings to the health care system. DenerveX is not yet commercially available.
DenerveX system is not yet CE marked or FDA cleared and is not yet commercially available.
About Medovex
Medovex was formed to acquire and develop a diversified portfolio of potentially ground breaking medical technology products. Criteria for selection include those products with potential for significant improvement in the quality of patient care combined with cost effectiveness. The Company’s first pipeline product, the DenerveX device, is intended to provide long lasting relief from pain associated with facet joint syndrome at significantly less cost than currently available options. To learn more about Medovex Corp., visit www.medovex.com
Safe Harbor Statement
Certain statements in this press release constitute “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “might,” “will,” “should,” “believe,” “expect,” “anticipate,” “estimate,” “continue,” “predict,” “forecast,” “project,” “plan,” “intend” or similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While the Company believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based on information available to us on the date of this release. These forward looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties, including without limitation those set forth in the Company’s filings with the Securities and Exchange Commission (the “SEC”), not limited to Risk Factors relating to its patent business contained therein. Thus, actual results could be materially different. The Company expressly disclaims any obligation to update or alter statements whether as a result of new information, future events or otherwise, except as required by law.