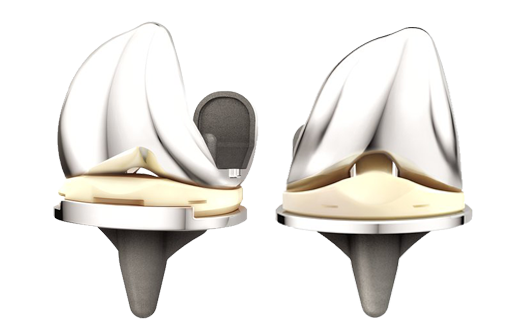
WARSAW, Ind., May 10, 2018 /PRNewswire/ — DePuy Synthes*, part of the Johnson & Johnson Medical Devices Companies**, today announced clinical findings outlining the potential benefits patients may experience when undergoing knee replacement with the ATTUNE Knee System.
First, a study1 presented at the 2nd World Arthroplasty Congress in Rome on Patient Reported Outcome Measures (PROMS) reported a statistically significant improvement in knee physical function at six months with the ATTUNE Knee compared to preoperative baseline, using the Knee injury and Osteoarthritis Outcome Score-Physical Function Short Form (KOOS-PS). The multicenter study of 200 cases from The Netherlands found that 80 percent of those ATTUNE Knee patients’ KOOS-PS improvements were realized by six months, with statistically significant improvements observed at intervals prior to six months. Also, pain and other PROMS were statistically significantly better at six weeks compared with pre-operative baseline.
“This multi-center outcomes study is further evidence of the positive performance of the ATTUNE Knee, and this study provides additional evidence on the rate of recovery – information that is useful when counseling patients before surgery,” said Geert Meermans, MD, Bravis Hospital, The Netherlands. “From my intraoperative and patient follow up experiences, the ATTUNE Knee appears to provide greater stability than other implants I have used, which may be a contributing factor to these positive results.”
Secondly, a report2 generated by DePuy Synthes summarized a series of studies across several countries with differing healthcare systems. This report, entitled “The Impact of Implant Design on Hospital Length of Stay and Discharge Destination: Evidence Summary Report,” evaluated the connection between implant design and patient hospital length of stay (LOS), and encompassed some data that has been presented or accepted at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).3-7 This retrospective review of five real-world evidence studies, conducted by DePuy Synthes in the US, UK, Germany, Italy and The Netherlands, was designed to evaluate whether patients treated with the ATTUNE Knee had a shorter hospital length of stay (LOS) versus a number of comparative implants. The report concluded that in each of these studies, the ATTUNE Knee patients were discharged from the hospital sooner than with the comparative implants used.3-7
Reducing LOS is recognized as an effective way to lessen the financial burden of elective orthopaedics by reducing resource utilization, thereby lowering the overall cost of care.8 In addition, reducing LOS may also positively impact patient satisfaction. Emerging evidence detailed in this report2 suggests the ATTUNE Knee may facilitate earlier hospital discharge, with these studies observing a reduced length of stay between 0.19 and 4 days on average, depending on the comparative implants/countries compared against.3-7 While differences in healthcare systems limit the direct comparison of these observed statistically significant improvements in LOS, this report highlights the positive impact the ATTUNE Knee may deliver in various rehabilitation settings.
Commenting on the LOS findings to which his Center also contributed, Dr. Meermans concluded, “While it’s important to acknowledge that many factors can contribute to differences in length of stay, the data seems to suggest that implant design may be an important factor to consider in total knee arthroplasty procedures. This latest evidence gives me greater confidence that the device could help hospitals cost-effectively keep pace with the growing total knee arthroplasty demand.”
These findings come at a time when healthcare systems are facing unprecedented challenges due to demographic shifts and increases in demand due to the predicted global growth of knee replacement procedures.9 It is increasingly important for healthcare providers to demonstrate improved clinical, economic and patient reported outcomes to help minimize the total cost per procedure while maintaining and, where possible, improving quality of care.
Torbjorn Skold, Vice President, DePuy Synthes EMEA Joint Reconstruction added: “I’m delighted that this data demonstrated that study patients receiving the ATTUNE Knee experienced positive outcomes related to the rate of return of functional outcomes and reduced length of stay, allowing these patients to get back to a positive quality of life.”
Notes to Editors
About the Length of Stay (LOS) Report “The Impact of Implant Design on Hospital Length of Stay and Discharge Destination: Evidence Summary Report”2
The purpose of this report was to summarize a series of 5 studies that were designed to evaluate whether patients treated with the ATTUNE Knee had a shorter LOS versus certain comparative implants. The studies were conducted in several countries with differing healthcare systems. All the studies in this report defined LOS as the primary endpoint. An additional study by Clement et al., (2017) conducted in Scotland also observed a statistically significant 1.2-day reduction (P<0.001) in LOS versus another leading knee system but is not included in this report as LOS was not the primary outcome.10
There are several important aspects of this report that limit the comparability of results among the studies and the generalizability to other institutions/countries. Due to factors within each country’s healthcare systems, baseline mean LOS will vary, which limits direct comparison of the means and differences between studies. Statistical analyses also differ by study as they controlled for different factors because of the feasibility of retrospective chart review/data collection at each site, which further limits the comparability of the results. This review however, was not intended to aggregate the data, it was to report each study in isolation and highlight the reductions in LOS seen in multiple healthcare settings following the adoption of the ATTUNE Knee.
Study summary
Study 1 examined insurance claims data across a range of US geographies and institution types (38 hospitals) including 1,178 US patients who received the ATTUNE Knee and 5,707 patients who received the Triathlon® Knee, with the patients receiving the ATTUNE Knee having a shorter average LOS (<0.19 day) compared to the Triathlon® Knee patients. ATTUNE Knee patients were also 39% less likely to be discharged to a skilled nursing facility.3
Study 2 evaluated patients at a University Teaching Hospital in the UK, 238 of whom were implanted with an ATTUNE Knee, 332 with a SIGMA Knee and 149 with a Columbus® Knee. Patients implanted with the ATTUNE Knee on average experienced a 0.8-day shorter LOS compared to SIGMA Knee patients and a 1-day reduction compared to patients treated with the Columbus® Knee. Both comparisons were statistically significant.4
Study 3 evaluated patients at a private German hospital, 85 of whom were implanted with the ATTUNE Knee compared to 85 implanted with the LCS Complete Knee System. Patients in this study implanted with the ATTUNE Knee were discharged from hospital on average 2.1 days earlier than patients implanted with the LCS Complete Knee. This reduction in LOS was statistically significant.5
Study 4 evaluated two groups at a private Italian hospital: 100 patients implanted with the ATTUNE Knee and 100 patients who received a SIGMA Knee. A statistically significant difference in LOS was observed: the patients implanted with an ATTUNE Knee were discharged from the hospital on average 4 days earlier than the patients implanted with a SIGMA Knee. 6
Study 5 evaluated two groups at a public hospital in The Netherlands: 100 patients implanted with the ATTUNE Knee and 100 patients implanted with the SIGMA Knee. Both groups were treated with the same rehabilitation protocol. Patients in this study who were implanted with the ATTUNE Knee were discharged from the hospital on average 0.67 days earlier than patients implanted with the SIGMA Knee. Similar to study 1,3 ATTUNE Knee patients were also less likely to be discharged to a rehabilitation center (referred to as a skilled nursing facility in the US). For Study 5, the statistically significant improvements were achieved in a hospital environment with an established enhanced recovery program and a low baseline LOS.7
All the studies defined LOS as the primary endpoint. A copy of the full report can be found on ATTUNEevidence.com.
About the Johnson & Johnson Medical Devices Companies
The Johnson & Johnson Medical Devices Companies’ purpose is to reach more patients and restore more lives. Having advanced patient care for more than a century, these companies represent an unparalleled breadth of products, services, programs and research and development capabilities in surgical technology, orthopaedics, interventional solutions and specialty surgery with an offering directed at delivering clinical and economic value to health care systems worldwide.
About DePuy Synthes
DePuy Synthes, part of the Johnson & Johnson Medical Devices Companies, provides one of the most comprehensive orthopaedics portfolios in the world. DePuy Synthes solutions, in specialties including joint reconstruction, trauma, craniomaxillofacial, spinal surgery and sports medicine, are designed to advance patient care while delivering clinical and economic value to health care systems worldwide. For more information, visit www.depuysynthes.com.
*DePuy Synthes represents the products and services of DePuy Synthes, Inc. and its affiliates.
**The Johnson & Johnson Medical Devices Companies comprise the surgery, orthopaedics, and interventional solutions businesses within Johnson & Johnson’s Medical Devices segment.
The third-party trademarks used herein are the trademarks of their respective owners.
© DePuy Synthes 2018. All rights reserved.
References
- van Loon C, Meermans G, Baas N, Vergroesen D, van Kampen P, Dwyer K, Lesko J, Huey V. Early Recovery Rate After A New Design Total Knee Arthroplasty (TKA): A Prospective, Multicenter Cohort of 200 Cases. Poster Accepted to World Arthroplasty Congress April 19-21, 2018. Rome, Italy, Poster # P101.
- The Impact of Implant Design on Hospital Length of Stay and Discharge Destination Evidence Summary Report. 090088-180413. Available at ATTUNEevidence.com. Accessed April 2018.
- Etter K, Lerner J, Kalsekar I, de Moor C, Yoo A, Swank M. Comparative Analysis of Hospital Length of Stay and Discharge Status of Two Contemporary Primary Total Knee Systems. The Journal of Knee Surgery. 2017 Aug 25.
- Mantel J, Corso KA, Wei D, Holy CE, Muehlendyck C, Jayakumar P, Higgins M, Westbrook A. Economic Effectiveness Of The ATTUNE® Knee System-Analysis Of Real World Hospital Length Of Stay And Incidence Of Early Complications. Value in Health. 2017 Oct 1;20(9): A579.
- Brüggenjürgen B, Muehlendyck C, Gador L, Katzer A. Length of Hospitalisation After ATTUNE® Knee Joint Arthroplasty (TKA)-Results of A German Retrospective Database Analysis. Value in Health. 2017 Oct 1;20(9):A597.
- Pipino G, Paragò V, Corso KA, Wigham R, Holy CE, Do Rego B. Economic Outcomes Of The ATTUNE® Knee System: Analysis Of Real World Length Of Stay In An Italian Hospital. Value in Health. 2017 Oct 1;20(9):A595.
- Meermans G, Galvain T, Wigham R, Do Rego B, Schröer D. Comparative analysis investigating the impact of implant design on hospital length of stay and discharge destination in a Dutch hospital with an established enhanced recovery program. DSEM/JRC/0118/0984. Poster Accepted to ISPOR Annual Meeting May 19-23, 2018. Baltimore US. Presentation code PMD23.
- Briggs TWR. A national review of adult elective orthopaedic services in England: Getting it right first time. Available at: https://www.boa.ac.uk/wp-content/uploads/2015/03/GIRFT-National-Report-Mar15..pdf. Accessed April 2018.
- Patel A, Pavlou G, Mújica-Mota RE, Toms AD The epidemiology of revision total knee and hip arthroplasty in England and Wales: a comparative analysis with projections for the United States. A study using the National Joint Registry dataset. Bone & Joint J. 2015 Aug;97-B(8):1076-81.
- Clement N, Brenkel I, Walmsley P. IMPROVED EARLY FUNCTIONAL OUTCOME WITH THE ATTUNE TOTAL KNEE REPLACMENT: A PROPENSITY SCORE MATCHED TRIAL. 2017. Poster session presented at British Association for Surgery of the Knee, Southport, United Kingdom.
Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding the ATTUNE Knee System*. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of DePuy Synthes, any of the other Johnson & Johnson Medical Devices Companies and/or Johnson & Johnson. Risks and uncertainties include, but are not limited to: uncertainty of commercial success; challenges to patents; competition, including technological advances, new products and patents attained by competitors; manufacturing difficulties and delays; product efficacy or safety concerns resulting in product recalls or regulatory action; changes to applicable laws and regulations, including global health care reforms; changes in behavior and spending patterns of purchasers of health care products and services; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s Annual Report on Form 10-K for the fiscal year ended December 31, 2017, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in the company’s subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at www.sec.gov, www.jnj.com or on request from Johnson & Johnson. Neither DePuy Synthes, the Johnson & Johnson Medical Devices Companies nor Johnson & Johnson undertakes to update any forward-looking statement as a result of new information or future events or developments.
*DePuy Synthes represents the products and services of DePuy Synthes, Inc. and its affiliates.
091374-180507
SOURCE DePuy Synthes
Related Links
http://www.depuysynthes.com