October 18, 2017
NEW YORK: On August 2, 2017, an American surgical team conducted a surgery to replace the sternum and partial ribcage of a 20 year-old woman using a custom 3D-printed composite titanium/porous polyethylene implant. It is the first time this technology has been used in the United States and only the second time in the world that a 3D-printed composite sternum and ribcage has been implanted.
The implant was designed and created for a patient named Penelope Heller by Anatomics, an Australian company that specializes in the manufacture of patient specific implants for surgeons around the world, and was made available to the patient via the United States Food & Drug Administration’s (FDA) Expanded Access (Compassionate Use) Program. Anatomics’ custom 3D printed implants do not have marketing approval in the US. The FDA’s Expanded Access Program provides a path to accessing devices for patients whom the treating physician believes may provide a benefit, but have not received FDA marketing approval.
The surgical team, led by Jeffrey L. Port M.D., attending cardiothoracic surgeon at NewYork-Presbyterian/Weill Cornell Medical Center and professor of clinical cardiothoracic surgery at Weill Cornell Medicine, undertook the operation to revise a prior sternum/ribcage removal and reconstruction conducted in 2014. The patient underwent the original procedure to remove a malignant bone tumor after she was diagnosed with chondrosarcoma, a rare cancer affecting the bones and joints that is resistant to chemotherapy and radiotherapy and requires surgical removal.
In the original resection surgery, the cancer was successfully removed and the previous surgeon created an implant for the patient, using off-the-shelf Gore-Tex (low-density porous polytetrafluoroethylene, or PTFE) and Bone Cement (methyl methacrylate, or MMC). Afterward, the patient was cancer-free but continued to experience pain and issues with breathing that did not improve with time. Online research led the patient to a similar surgery performed in Spain using a custom implant also developed by Anatomics. The patient and her family then worked with the staff at NewYork-Presbyterian/Weill Cornell Medical Center and Anatomics to access the implant under the FDA’s Expanded Access program.
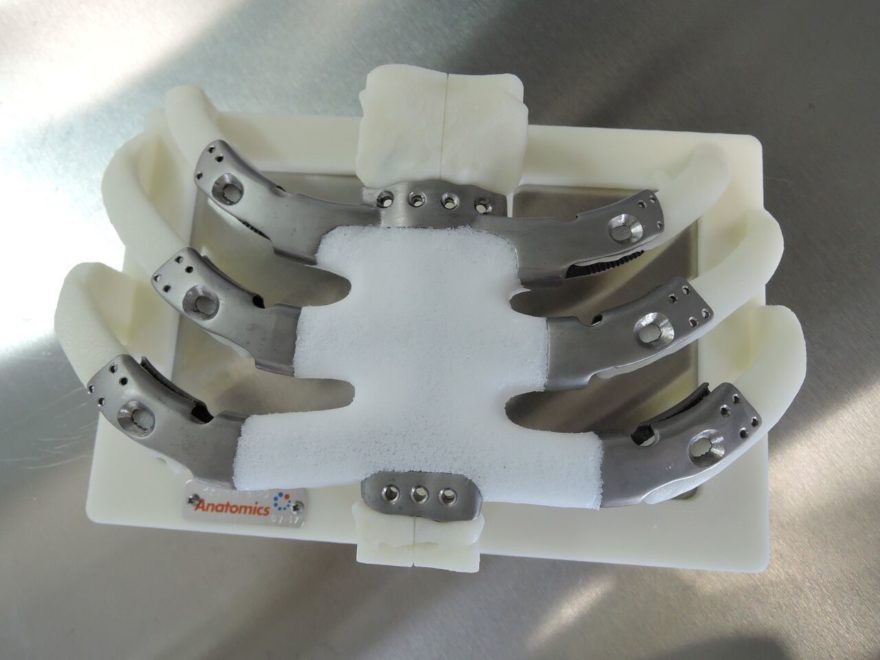
The custom implant was designed using high-resolution CT scans of the patient’s chest that were sent to Anatomics engineers via the secure AnatomicsRx software platform. After Dr. Port reviewed and confirmed the design on-line with the engineers, a biomodel of the patient’s sternum and ribcage was created and a build code was sent to Australia’s Commonwealth Scientific & Industrial Research Organisation’s (CSIRO) 3D printing laboratory, Lab 22. The patient’s custom sternum implant is the second in the world to also use Anatomics’ PoreStar technology, a proprietary porous polyethylene material with bone-like architecture. PoreStar is currently awaiting FDA marketing approval.
The surgical team, led by Jeffrey L. Port M.D., attending cardiothoracic surgeon at NewYork-Presbyterian/Weill Cornell Medical Center and professor of clinical cardiothoracic surgery at Weill Cornell Medicine, undertook the operation to revise a prior sternum/ribcage removal and reconstruction conducted in 2014. The patient underwent the original procedure to remove a malignant bone tumor after she was diagnosed with chondrosarcoma, a rare cancer affecting the bones and joints that is resistant to chemotherapy and radiotherapy and requires surgical removal.
In the original resection surgery, the cancer was successfully removed and the previous surgeon created an implant for the patient, using off-the-shelf Gore-Tex (low-density porous polytetrafluoroethylene, or PTFE) and Bone Cement (methyl methacrylate, or MMC). Afterward, the patient was cancer-free but continued to experience pain and issues with breathing that did not improve with time. Online research led the patient to a similar surgery performed in Spain using a custom implant also developed by Anatomics. The patient and her family then worked with the staff at NewYork-Presbyterian/Weill Cornell Medical Center and Anatomics to access the implant under the FDA’s Expanded Access program.
The custom implant was designed using high-resolution CT scans of the patient’s chest that were sent to Anatomics engineers via the secure AnatomicsRx software platform. After Dr. Port reviewed and confirmed the design on-line with the engineers, a biomodel of the patient’s sternum and ribcage was created and a build code was sent to Australia’s Commonwealth Scientific & Industrial Research Organisation’s (CSIRO) 3D printing laboratory, Lab 22. The patient’s custom sternum implant is the second in the world to also use Anatomics’ PoreStar technology, a proprietary porous polyethylene material with bone-like architecture. PoreStar is currently awaiting FDA marketing approval.
“After my initial resection and reconstruction surgery, I continued to experience breathing issues and pain,” said Ms. Heller. “With a long, active life ahead of me, I wanted to participate in activities that I love fully and without pain. Electing to have this procedure was a big decision, and I’m coming forward to empower other people in the same position.”
Anatomics’ Executive Chairman Paul D’Urso, MBBS (Hons) Ph.D. FRACS said, “Anatomics is humbled by the strength of the thousands of patients we have helped over 25 years since inventing BioModeling technology. The patient’s story is one of many, but what makes it truly remarkable is how the patient and her family, Dr. Port and the staff at NewYork-Presbyterian/Weill Cornell, Anatomics, and the FDA came together to make this story a reality. It was a group effort that began with the patient’s pursuit of information.”
About Anatomics
Anatomics is an Australian medical device company that has been manufacturing and marketing surgical products to surgeons locally and internationally since 1996. Anatomics pioneered CT scan derived surgical implant technology and was first to market with an innovative, quality product that assists surgeons to produce better surgical outcomes and reduce expensive operating time. For more information, visit http://www.anatomics.com and follow the company on twitter at https://twitter.com/anatomicsrx.
For further information contact:
(Media) Tiberend Strategic Advisors, Inc
Janine McCargo, Senior Vice President
Telephone: +1 (646) 604-5150
e-mail: jmccargo@tiberend.com
Anatomics United States
Dr. Dean Carson, Vice President US Operations
951 Mariners Island Blvd. San Mateo, CA 94404 (CSIRO Office)
Telephone: +1 (415) 806-2599
e-mail: dean.carson@anatomics.com
Anatomics Australia
Dr. Paul D’Urso, Executive Chairman
Suite 1, 23-27 Wellington Street, St Kilda, Victoria, 3182, Australia
Telephone: +61 3 9529-8088
e-mail: contact@anatomics.com