ATLANTA, GA — (Marketwired) — 08/21/17 — Medovex Corp. (NASDAQ: MDVX) (“Medovex” or the “Company“), the developer of the DenerveX™ System, a new and novel device designed for enduring relief of Facet Joint Syndrome related to back pain, today announced it has appointed Jesse Crowne as Director and Executive Co-Chairman of the Board.
Jesse Crowne has been acting as a Vice President of Business Development for the Company since January 2015. Mr. Crowne has been a Managing Partner at Gorlin Companies; a healthcare focused single family office specializing in founding and funding early ventures since July 2015. Crowne replaces Steve Gorlin who will remain with the Company in a consultancy role.
Between August 2015 and January 2017, Mr. Crowne was the President of Vavotar Life Sciences, a private clinical stage biotechnology company developing antibody directed oncology products. Since 2016, Mr. Crowne has served as an Adjunct Professor at Westminster College teaching a course on financing new ventures to MBA students. From October 2013 to March 2014, he was the Co-Founder of Virtual Clinic Trials, LLC, a cloud based document management solution for clinical trials until it was sold to Global Deal Market in 2014. From 2010 to June 2014, he was an associate at White Pine Medical, a subsidiary of Essex Woodlands, which was a private equity investment fund seeking late stage medical device opportunities.
Steve Gorlin, Medovex Co-founder, stated, “I’m pleased to have accomplished my initial goal of seeing Medovex successfully progress from the development stage to the commercialization phase. I’m also extremely proud of our entire management team and their collective effort in getting us to the opportunistic place we find ourselves today. At no point in the Company’s history have I ever been more confident in its future. Over the years, I’ve been intimately involved in many small healthcare related companies which eventually went on to experience sizeable value creation, but few were as personal as Medovex. My continuing commitment to the company can be no better evidenced than by my Form 4 filing made Wednesday memorializing my open market purchase of an additional 200,000 shares of our stock.”
Gorlin continued, “I’ve worked closely with Jesse Crowne at my own company for some time now and I handpicked him based on a steadfast confidence in his ability to finish what we’ve started. I view him as the ideal person to assist in taking Medovex to the next level and I look forward to continuing to work closely with him, the board and management as we together seek to accomplish my ultimate goal of maximizing shareholder value.”
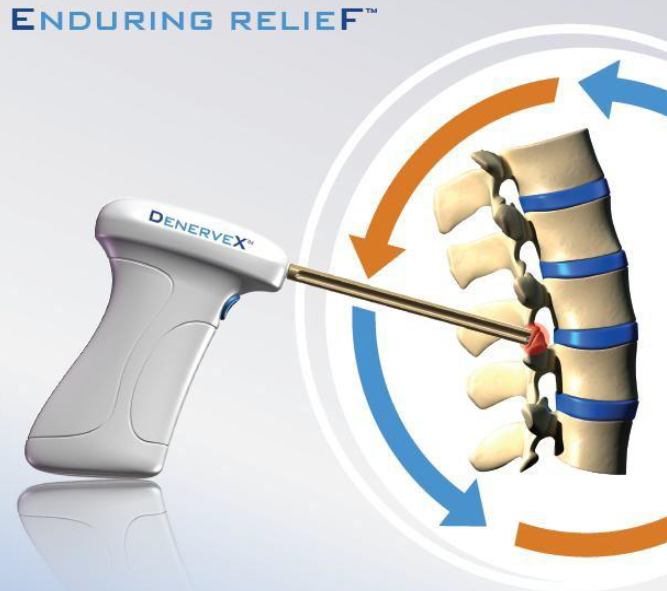
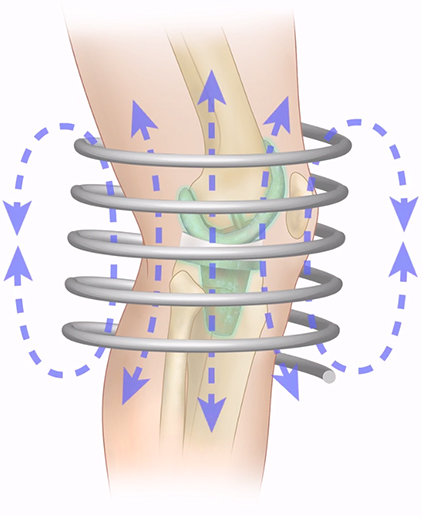
The Company’s DenerveX System recently received CE Mark approval and clearance for commercialization in the European countries and offers a unique way to perform a Facet Joint Syndrome treatment.
Facet Joint Syndrome (FJS), also known as spinal osteoarthritis, spinal arthritis, or facet joint osteoarthritis, is a significant health and economic problem in the United States and other countries in the EU and Rest of World affecting millions each year. Current treatment options are generally temporary and there is no proven long-lasting option for FJS.
The DenerveX System is a highly differentiated technology. It denervates and removes capsular tissue from the Facet Joint in one single procedure. Treatment results from the combined effect of a deburring or polishing action and RF ablation treatment on the Facet Joint. Using this new technique, the slowly rotating burr removes the targeted facet joint synovial membrane and joint surface while the heat ablation destroys tissue and denudes any residual nervous and synovial membrane overlying the joint, removing the end point sensory tissue of the joint.
The DenerveX System consists of the DenerveX Kit which contains the DenerveX Device, a single use medical device and the DenerveX Pro-40 Power Generator. DenerveX system is not yet FDA cleared.
About Medovex
Medovex was formed to acquire and develop a diversified portfolio of potentially ground breaking medical technology products. Criteria for selection include those products with potential for significant improvement in the quality of patient care combined with cost effectiveness. The Company’s first pipeline product, the DenerveX device, is intended to provide long lasting relief from pain associated with facet joint syndrome at significantly less cost than currently available options. To learn more about Medovex Corp., visit www.medovex.com
Safe Harbor Statement
Certain statements in this press release constitute “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “might,” “will,” “should,” “believe,” “expect,” “anticipate,” “estimate,” “continue,” “predict,” “forecast,” “project,” “plan,” “intend” or similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. While the Company believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based on information available to us on the date of this release. These forward looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties, including without limitation those set forth in the Company’s filings with the Securities and Exchange Commission (the “SEC”), not limited to Risk Factors relating to its patent business contained therein. Thus, actual results could be materially different. The Company expressly disclaims any obligation to update or alter statements whether as a result of new information, future events or otherwise, except as required by law.
CONTACT INFORMATION
Medovex Corp.
Jason Assad
470-505-9905
Email Contact