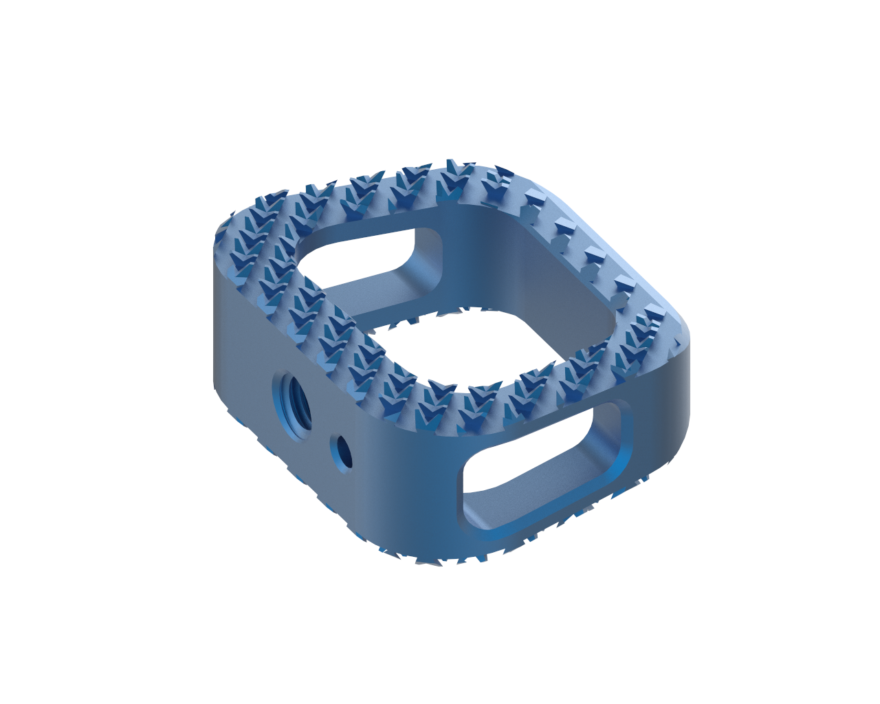
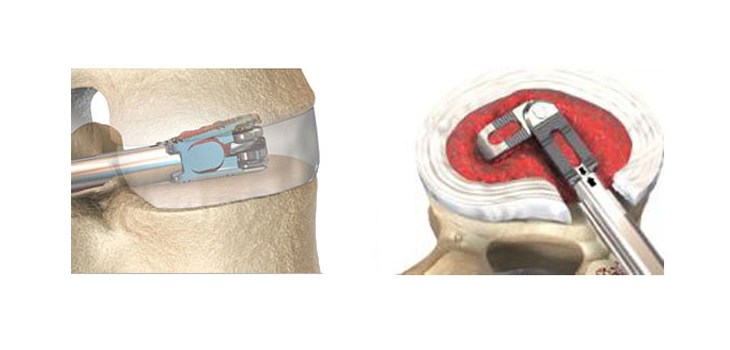
DALLAS, TX – 07/25/2017 (PRESS RELEASE JET) — CTL Medical Corporation, a Dallas-based medical device manufacturing and service company, has secured FDA clearance to market its new MATISSE™ Titanium ACIF Cage implant with TiCro™ surface, used in the practice of spine fusion surgery.
The MATISSE™ Titanium ACIF Cage with CTL Medical’s proprietary TiCro™ surface technology offers 200 percent greater endplate contact surface area, as well as bone conforming geometry for increased mechanical locking at the cage and bone interface. The implant includes a tapered leading edge for easy insertion and a large graft area to further promote bony fusion. The device is available in a variety of sizes and configurations to accommodate variations in vertebral levels and patient anatomy.
“Countless engineering hours and R&D resources went in to developing CTL Medical’s patent pending TiCro™ surface technology, which was designed to expand our current portfolio, advance fusion technologies, and offer spine surgeons numerous surgical advantages,” stated Danny Chon, president & CEO of CTL Medical Corporation. “The Matisse™ system includes streamlined instrumentation and a variety of footprints, heights and lordotic profiles to accommodate variations in patient anatomy.”
MATISSE™ Titanium ACIF Cage with TiCro™ is indicated for use in skeletally mature patients with degenerative disc disease (DDD) of the cervical spine with accompanying radicular symptoms at one-disc level. DDD is defined as discogenic pain with degeneration of the disc confirmed by patient history and radiographic studies. MATISSE Anterior Cervical Interbody Fusion Cage system is used to facilitate intervertebral body fusion in the cervical spine at the C3 to C7 disc levels using autograft bone. MATISSE™ Titanium ACIF Cage with TiCro™ is to be used with supplemental fixation, such as CTL Medical’s VAN GOGH™ Anterior cervical plating system, which has been cleared for use in the cervical spine.
The use of cage devices in spinal surgery began with clinical trials in 1989, and since then, multiple implant improvements have debuted – leading to easier procedures, benefitting both spine surgeons and overall patient success and recovery times.
For more information on CTL Medical Corporation, visit www.ctlmed.com.
About CTL Medical Corporation
CTL Medical Corporation is a fully integrated, industry-leading, global medical device design, development and manufacturing company. CTL has assembled a world-class executive team, bringing together some of the industry’s most exceptional talent, positioning it to be a leader in medical device design and manufacturing. For more information, visit www.ctlmed.com.
Jeff Cheatham
TrizCom PR
O: 972-247-1369
C: 972-961-6171
jeffc@trizcom.com
Media Contacts:
Company Name: TrizCom PR
Full Name: Jeff Cheatham
Phone: (972) 247-1369
Email Address: SEND EMAIL
Website: www.trizcom.com