SACRAMENTO, CALIF. (PRWEB) JANUARY 17, 2018
Molecular Matrix, Inc (MMI) today announced the FDA clearance (510k) of Osteo-P™, a value-driven, effective musculoskeletal solution for bone regeneration.
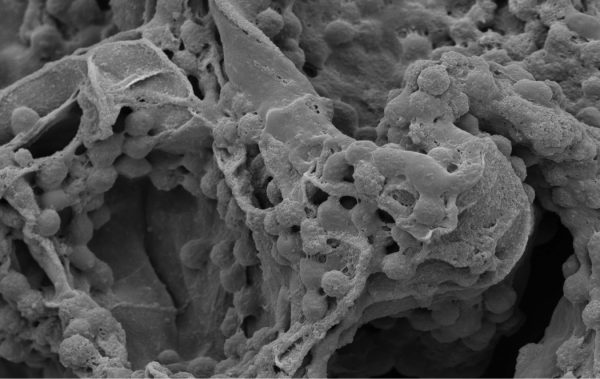
The Osteo-P™ bone graft substitute is a non-mineralized, synthetic bone void filler made of a hyper-crosslinked carbohydrate polymer (HCCP). It is highly porous, biocompatible, biodegradable, and has shown exceptional capabilities of bone repair.
The polymer technology offers several advantages over current bone graft substitutes, including exceptional bone formation and implant resorption, real-time fusion monitoring, and the ability to hold a suture.
Based on proprietary research, Osteo-P™ is intended for the filling of bone defects created surgically or through traumatic injury. When placed or gently packed into bone voids, Osteo-P™ supports and guides the ingrowth of new bone across the graft site, after which it is resorbed and replaced by newly formed bone during the healing process.
“Osteo-P™ provides an optimal microenvironment for infiltration of bone precursors such as osteoblasts that have been known to play a key role in bone regeneration,” says Molecular Matrix founder and CEO Charles Lee, PhD. “This microenvironment includes a significant surface area that allows the flow of fluids and metabolites, leading to the formation of healthy bone.”
The product is available in large pore granules, sheets, cubes, wedges and cylinders offering greater application, flexibility, and excellent handling characteristics. Additionally, customized products are routinely manufactured for collaborative research projects and may be useful for complex anatomic defects.
“The compressibility and tensile strength of Osteo-P™ allows the scaffold to be gently packed in to fill a void.” says Kee D. Kim, MD, Professor of Neurological Surgery and Co-Director of the University of California Davis Health, Spine Center. “The degradation profile, in conjunction with host bone regeneration, results in the formation of the patient’s own healthy bone.”
Osteo-P™ is intended for single patient use only, and is provided in a one-time use, with double sterile packaging. It is not indicated for use as a structural support in load bearing applications. The technology is delivered in a ready to use sterile package, and stores at room temperature. Additionally, Osteo-P™ is not subject to degradation through hydrolysis, making shipping logistics more convenient.
“The Osteo-P™ bone void filler provides cutting edge technology for our sales channels, offering significant advantages over other bone void fillers in the market today. In addition, this technology platform ensures Molecular Matrix will continue to develop this technology for multiple other tissue regeneration initiatives and other specific clinical applications” states Jim Keefer, COO of Molecular Matrix. “We are all very excited about our future, and expect significant market penetration over the coming months and years.”
Molecular Matrix’s HCCP technology provides a broad platform for tissue engineering in the orthopedic disciplines. Its HCCP has been shown to be effective for bone regeneration, evidenced by bone precursor cell activities. The technology will bring advancements in orthopedics, spine, trauma, and dental applications with superior bone regeneration products.
MMI has assembled a strong management team and Board of Directors (BOD) and believes that the quality and experience of the group is a critical factor to the success of the Company. MMI’s management team and BOD have over 75 years of experience in research and development, spine and biologics, and commercialization of new technologies.