Posted by Dann Bruno
These days, wireless technology is increasingly becoming part of the nuts and bolts of innovative design.
From automobiles to oil wells, devices and machines are transmitting data wirelessly—information that measures performance, provides failure alerts, and helps the human users have a better experience.
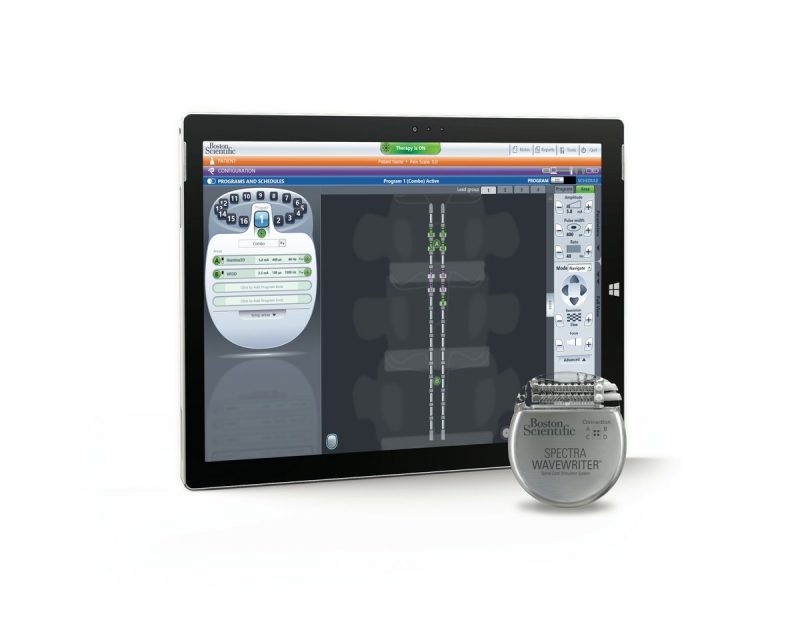
Now, Intellirod Spine adds to the list with implantable, wireless, monitoring systems designed to measure the progress of spine healing after lumbar surgery.
From inside the body, these sensors send physicians the data they need to achieve potentially better patient outcomes, both during surgery and post-operatively.
Better Data Contributes to Better Outcomes
Each year in the U.S., more than 450,000 lumbar spinal fusions are performed to treat fractures and instability, to correct deformities, or to eliminate pain. In these procedures, the surgeon positions bone grafts around the spine; the goal is for the body to grow new bone to connect (or fuse) the grafts to existing vertebrae.
During surgery, a system of metal rods and screws, typically made of titanium or cobalt chrome, is implanted to stabilize the spine and help it heal.
“Strain on the implanted rods lessens as new bone grows after surgery,” said Ric Navarro, president and CEO of Intellirod Spine. “When the spine doesn’t fuse, there is more strain on the rods.”
Currently, surgeons use radiography and CT scans during recovery and rehab to determine whether the spine is successfully fusing and growing new bone after surgery. Intellirod Spine’s wireless sensors provide mechanical data on the strain on the rods. This information compliments medical assessments from x-ray or CT scans.
“We are undergoing clinical studies to collect data for FDA at the Cleveland Clinic, the OhioHealth Grant Medical Center, and the Norton Leatherman Spine Center in Louisville and seeking a fourth site, said Navarro, who has more than 25 years of medical device and implant experience in artificial heart, operating room equipment, and spinal implants. He has also been inventor on 22 patents and has commercialized numerous spine and operating room products.