24 April 2018
Smith & Nephew (NYSE:SNN; LSE:SN), the global medical technology business, announces that Spire Bushey Hospital in the UK, has completed Europe’s first robotics-assisted total knee replacement procedure using the NAVIO◊ Surgical System.
Consultant Orthopaedic Surgeon Mr Richard Carrington, MBBS, FRCS (Orth), performed the first surgery using the Smith & Nephew JOURNEY◊ II Total Knee System.
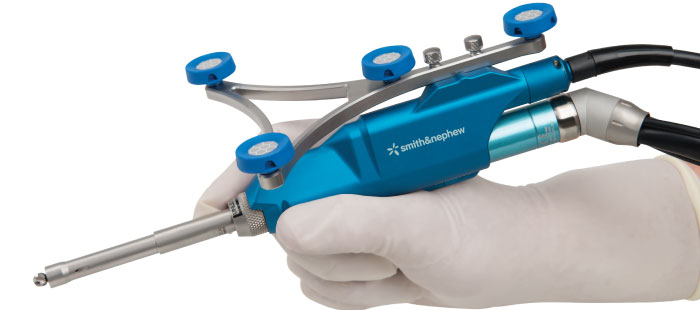
The Smith & Nephew NAVIO Surgical System is a next generation handheld robotics platform designed to aid surgeons with component positioning, ligament balancing and bone preparation – key factors that may drive implant survivorship.1,2 NAVIO is designed to help the surgeon with surgical planning and balancing of the joint to the patient’s specific needs. The handheld instrumentation uses multiple control modes to help the surgeon accurately prepare the bone for implantation.
The small footprint of NAVIO allows for easy set up and portability. Furthermore, NAVIO does not require a preoperative image, such as a CT scan. This allows patients to receive the benefits of robotics-assistance without the extra steps, costs, and radiation associated with additional preoperative imaging.3
“We are proud to support Spire Bushey in being the first hospital in Europe to bring patients the benefits of a robotic-assisted total knee procedure using the NAVIO Surgical System,” said Massimiliano Colella, President Europe and Canada, Smith & Nephew. “It is an important innovation as 80% of all knee replacement surgeries globally are total knees4, and it is exciting that Spire’s patients can now benefit from the improvements in accuracy that robotics is designed to deliver.”
Enquiries
Media
Dave Snyder
+1 (978) 749-1440
Smith & Nephew plc
References
About Smith & Nephew
Smith & Nephew is a global medical technology business dedicated to supporting healthcare professionals in their daily efforts to improve the lives of their patients. With leadership positions in Orthopaedic Reconstruction, Advanced Wound Management, Sports Medicine and Trauma & Extremities, Smith & Nephew has more than 15,000 employees and a presence in more than 100 countries. Annual sales in 2017 were almost $4.8 billion. Smith & Nephew is a member of the FTSE100 (LSE: SN, NYSE: SNN).
For more information about Smith & Nephew, please visit our corporate website www.smith-nephew.com, follow @SmithNephewplc on Twitter or visit SmithNephewplc on Facebook.com
About Spire Bushey Hospital
Working with leading consultants in their field, we have become the private hospital of choice for knee replacements in London and our region. Across both 2016 and 2017*, more knee replacements were carried out at Spire Bushey than any other private London hospital. Our team of expert physiotherapists provide comprehensive pre-habilitation programmes to help strengthen the limb before surgery to improve recovery. They use the onsite gym and hydrotherapy pool to support patient rehabilitation following surgery.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as “aim”, “plan”, “intend”, “anticipate”, “well-placed”, “believe”, “estimate”, “expect”, “target”, “consider” and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith & Nephew, these factors include: economic and financial conditions in the markets we serve, especially those affecting health care providers, payers and customers; price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers; competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith & Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith & Nephew’s most recent annual report on Form 20-F, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith & Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith & Nephew are qualified by this caution. Smith & Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith & Nephew’s expectations.
◊ Trademark of Smith & Nephew. Certain marks registered US Patent and Trademark Office.