April 12, 2018
ST. PAUL, Minn.–(BUSINESS WIRE)–Spineology Inc., an innovator in anatomy-conserving spine surgery, is excited to announce that John Chi, M.D., M.P.H, Associate Professor of Neurosurgery at Harvard Medical School and the Director of Neurosurgical Spinal Oncology at Brigham and Women’s Hospital in Boston MA, has presented the first 12-month outcomes data from Spineology’s SCOUT clinical trial. The results were presented at the recent annual meeting of the International Society for the Advancement of Spine Surgery (ISASS) in Toronto, Canada.
The SCOUT study (Spineology Clinical Outcomes Trial), conducted under an FDA-approved IDE protocol, is a prospective multicenter non-randomized performance goal investigation, designed to evaluate safety and effectiveness outcomes in instrumented lumbar interbody fusion procedures for the treatment of degenerative disc disease (DDD).
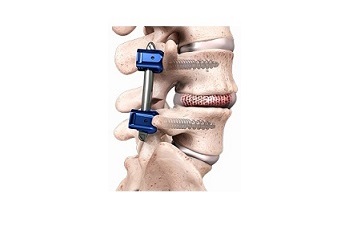
The fusion implant used in the SCOUT Study is Spineology’s porous graft containment mesh that deploys within the disc space as it is filled, permitting the packed bone graft to conform to the prepared vertebral body endplates. The system’s design allows for disc space preparation and implant placement through a small cannula.
The SCOUT trial includes 102 patients who were experiencing painful lumbar degenerative disc disease of at least six months’ duration. Patients are skeletally mature adults with single-level symptomatic lumbar DDD requiring interbody fusion. Patients have been enrolled at ten participating study sites. Dr. Chi and his co-authors reported on 84 treated subjects, with 60 completing 6-month follow-up and 29 completing 12-month follow-up. Study enrollment was completed in January of this year.
Statistically significant improvements were seen at both six months and twelve months in scores for low back pain and functional limitations, as recorded by Visual Analog Scale (VAS) and Oswestry Disability Index (ODI) respectively. At 12-month follow-up, all 29 subjects (100%) were fused, as read on CT by two independent radiologists. Twenty-seven of 29 subjects (93%) reported “Excellent” or “Good” satisfaction with their surgery. There were no serious device-related serious adverse events.
“These favorable interim results indicate the Spineology system’s potential to provide good arthrodesis and pain relief while minimizing tissue trauma,” said Dr. Chi. “We have seen shorter operating times, less blood loss, shorter hospital stays and excellent satisfaction for the patients treated in this trial.”
The list of nationwide study sites participating in the SCOUT IDE includes Brigham and Women’s Hospital, University of Vermont, the Spine Institute of Louisiana, Florida Orthopaedic Associates, and Georgetown University, among others. Details of the study may be found at the NIH clinical trials website, https://clinicaltrials.gov/ct2/show/NCT02347410?spons=spineology&rank=1.
Spineology’s OptiMesh® deployable implant received 510(k) clearance from FDA in 2003 for graft containment within the vertebral body. The SCOUT trial is designed to provide clinical data in support of a regulatory submission to FDA for expanded indications, allowing the company to market the implant to be used with bone graft and supplemental posterior fixation in support of lumbar interbody fusion for treating painful DDD.
“We are grateful to Dr. Chi and all the SCOUT investigators for their dedication in helping us to complete this important clinical trial,” said John Booth, Spineology CEO. “We look forward to completing the patient follow-up and gaining the market clearance that will allow us to market this unique fusion device to spine surgeons and their patients.”
About Spineology Inc.
Spineology Inc. provides innovative, anatomy-conserving spinal technologies for surgeons and their patients. Spineology surgical techniques conserve spinal bone, ligament and muscle tissue. Spineology is committed to increasing procedural efficiency, reducing surgical morbidity and accelerating patient recovery. Learn more at spineology.com.
Contacts
Spineology Inc.
John Booth, 651-256-8511
jbooth@spineology.com
or
Risdall
Dave Folkens, 651-286-6713
dave@risdall.com