25 January 2017
The arthroscopy market, which covers 39 countries and includes implant and capital equipment, is set to rise from $4.19 billion in 2016 to $5.73 billion by 2023, representing a compound annual growth rate (CAGR) of 4.6%, according to research and consulting firm GlobalData.
The company’s latest report states that this growth will be driven by the rising prevalence of sports injury, which is growing at a CAGR of 6.3% and is primarily caused by intense and repetitive training in major markets like the US and China. Other drivers include rising obesity rates, an aging population and the trend towards more innovative minimally-invasive techniques, reducing cost and recovery time for patients.
Tobe Madu, MSc, GlobalData’s Analyst covering Medical Devices, explains: “Sports medicine technology is built on minimally-invasive arthroscopic implants and equipment, and these will continue to drive the market, as well as other orthopedic segments. As both a technique-driven and an implant-based field, there are arthroscopic products continuously entering the market for difficult-to-access joints, and smaller implants for smaller repairs.
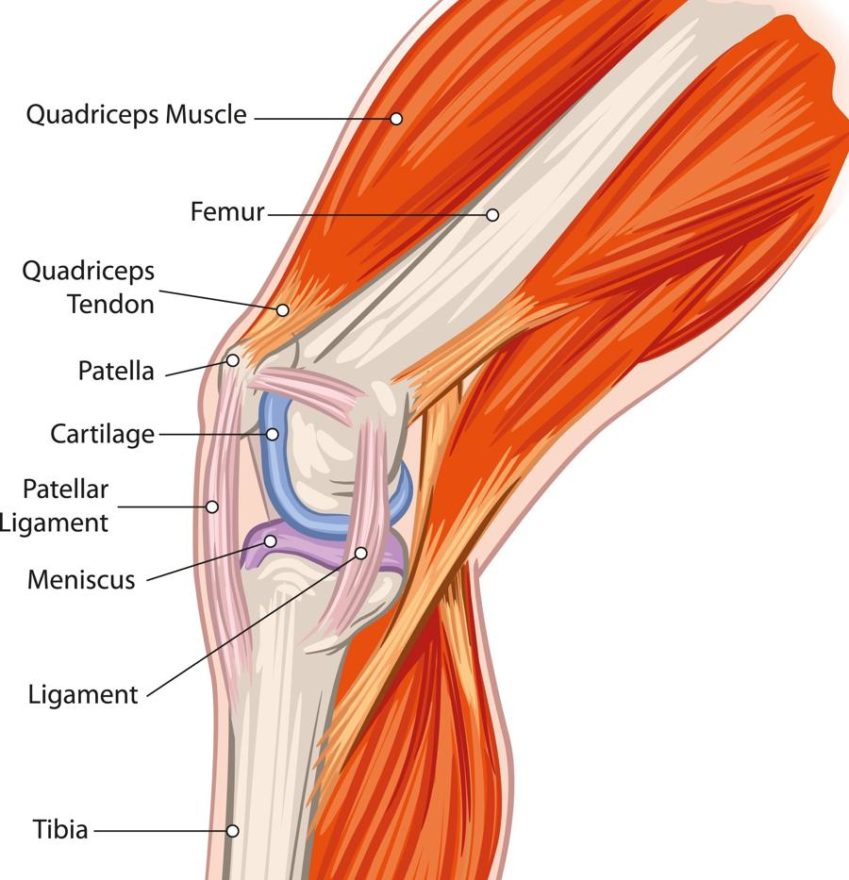
“Materials development has been a key interest for physicians, as they look for more durable and biocompatible implant materials. For example, knee cartilage injuries have driven the development of products for meniscal repair, while anterior cruciate ligament and posterior cruciate ligament injuries have inspired biocomposite and bioresorbable interference screws.”
While healthcare spending as reflected by reimbursement rates is expected to go down globally, the market outlook remains strong in western countries, as substantial growth in new indications will drive up procedure numbers. Meanwhile, countries such as Brazil, China, and India, will continue to see adoption of arthroscopic products as surgeon training improves and newer economical products are introduced in the market, according to GlobalData.
Madu continues: “As disposable surgical products turn into commodities, buyer decision will become increasingly price sensitive. Despite this development, market leaders such as Arthrex and Stryker, who maintain a culture of innovation and service while delivering cost-effective products, will stay ahead of competitors.
“The most significant area of growth is in hip arthroscopy, where global revenue is expected to increase by a CAGR of 14.5% during the forecast period.”
Editor’s notes
– Comments provided by Tobe Madu, MSc, GlobalData’s Analyst covering Medical Devices.
– Information based on GlobalData’s report: MediPoint: Sports Medicine – Global Analysis and Market Forecasts.
– This report was built using data and information sourced from proprietary databases, primary and secondary research, and in-house analysis conducted by GlobalData’s team of industry experts. The 39 major markets include the US, France, Germany, Italy, Spain, the UK, Japan, Brazil, China, India, South Korea, Australia, Canada, Mexico, Russia, Austria, Belgium, Czech Republic, Denmark, Finland, Greece, Hungary, Ireland, Netherlands, Norway, Poland, Portugal, Sweden, Switzerland, Turkey, Taiwan, New Zealand, Argentina, Chile, Egypt, Israel, Saudi Arabia, South Africa, and the United Arab Emirates.
– For guidelines on how to cite GlobalData, please see: https://healthcare.globaldata.com/media-center/quoting-globaldata
About GlobalData
GlobalData is a leading global research and consulting firm offering advanced analytics to help clients make better, more informed decisions every day. Our research and analysis is based on the expert knowledge of over 700 qualified business analysts and 25,000 interviews conducted with industry insiders every year, enabling us to offer the most relevant, reliable and actionable strategic business intelligence available for a wide range of industries.
For more information
Please get in contact if you have any questions about this or other GlobalData products. Analysts are available to comment. Contact the GlobalData press office +44 (0)161 359 5822 or email pr@globaldata.com