May 09, 2017
PLYMOUTH, Minn.–(BUSINESS WIRE)–Rotation Medical Inc., a medical device company focused on developing new technologies to treat rotator cuff disease, today announced key events for the upcoming Arthroscopy Association of North America Annual Meeting May 18-20 in Denver. These events include first-ever presentation of results of the REBUILD study of the company’s Bioinductive Implant for rotator cuff repair, as well as multiple podium presentations.
Rotation Medical’s REBUILD (Rotation MEdical BioindUctive ImpLant Database) is a prospective, non-randomized, real-world registry study designed to collect patient-reported outcomes, including shoulder function, pain and quality of life, after receiving the Bioinductive Implant. Dr. Louis McIntyre of Northwell Health Physician Partners Orthopaedic Institute at Sleepy Hollow (New York) will share results of the first 200 patients enrolled in the study, as well as provide case examples and an overview of published clinical data on Thursday, May 18, 11:40 a.m. to 12:30 p.m.
Attendees will also have the opportunity to learn more about the Rotation Medical Bioinductive Implant at the following events:
- Clinical Case Panel #1: Rotator Cuff. Dr. Jeffrey Abrams of Princeton Orthopaedic Associates (New Jersey) will highlight his use of the Bioinductive Implant. Thursday, May 18, 10:20-10:50 a.m.
- Symposium 1: The Failed Cuff, What Now? Dr. Michael O’Brien of Tulane Institute of Sports Medicine (Louisiana) will discuss patch options and results. Thursday, May 18, 10:50-11:30 a.m.
- The Holy Grail: Can We Successfully Treat the Overhead Athlete? Expert Advice and Experience. Dr. Michael T. Freehill of Michigan Medicine Orthopaedic Sports Medicine will highlight his use of the Bioinductive Implant. Thursday, May 18, 4:45-6:15 p.m.
- State of the Art Grafts and Patches in Rotator Cuff Surgery: Augmentation, Interpositions, Superior Capsular Reconstruction and Bioinductive Implant. Dr. Richard Ryu of the Ryu Hurvitz Orthopedic Clinic (California) and Dr. Matthew T. Provencher of The Steadman Clinic and Steadman Philippon Research Institute (Colorado) will present the biology and mechanics of rotator cuff patches and grafts, including the surgical technique for the Bioinductive Implant. Friday, May 19, 7:45-9:15 a.m.
- Feature Lecture #8: Patch Options and Results. Dr. Ryu will also provide an overview of the Bioinductive Implant for full thickness and partial tears. Friday, May 19, 3:15-3:30 p.m.
“Given the high failure rate and difficult recovery required for traditional approaches to rotator cuff repair, we believe the results of our REBUILD real-world study will speak to the potential of our Bioinductive Implant to transform the treatment of rotator cuff disease,” said Martha Shadan, president and CEO of Rotation Medical. “Attendees at this year’s AANA Annual Meeting will also have many opportunities to hear from their peers how our Bioinductive Implant can improve outcomes for people with rotator cuff tears.”
About Rotator Cuff Tears
Rotator cuff damage is the most common source of shoulder pain, affecting more than 4 million people annually in the U.S. Traditional approaches to treating degenerate or torn rotator cuffs often do not address the poor quality of the underlying tendon tissue, and a significant number of these tendons, after standard treatment, either degenerate further and/or re-tear.
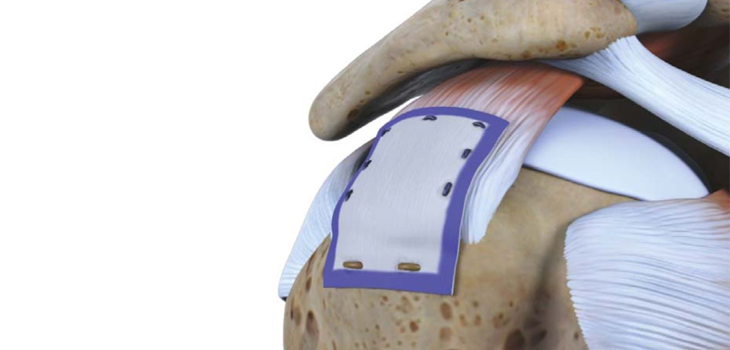
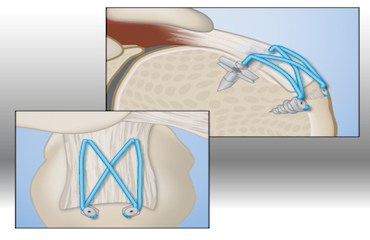
About the Rotation Medical BioInductive Implant
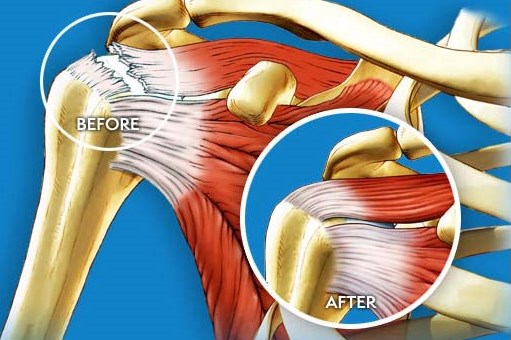
Cleared by the U.S. Food and Drug Administration in March 2014, the Rotation Medical Bioinductive Implant is designed to address both the biomechanics and biology required to heal a rotator cuff tendon tear by inducing new tissue growth at the site of implantation, resulting in increased tendon thickness and healing of tendon defects with new tissue growth. The collagen-based implant is about the size of a postage stamp and it is part of the Rotation Medical rotator cuff system, which also includes disposable instruments that allow the arthroscopic procedure to be performed easily and quickly. For important safety information, visit http://rotationmedical.com/our-solution/risks/.
About Rotation Medical
Rotation Medical Inc. was founded in 2009 and is committed to improving the treatment of rotator cuff disease with the Rotation Medical rotator cuff system, a breakthrough technology that has the potential to prevent rotator cuff disease progression and reduce re-tears by inducing the growth of new tendinous tissue. The company is privately held and funded by New Enterprise Associates (NEA), Life Sciences Partners (LSP) and Pappas Ventures. For more information, visit http://www.rotationmedical.com/.
Contacts
Merryman Communications
Joni Ramirez
323-532-0746
joni@merrymancommunications.com