September 27, 2016 – ERAGNY-SUR-OISE, France–(BUSINESS WIRE)
SAFE ORTHOPAEDICS (Paris:SAFOR) (FR0012452746 – SAFOR), a company offering innovative ranges of sterile implants combined with their single-use instruments has announced its half-year 2016 results for the six months to June 30, 2016, as approved by the Board of Directors on September 27, 2016.
Pierre Dumouchel, Chief Executive Officer of Safe Orthopaedics, said: “In parallel with the implementation of our new strategy of refocusing on regions growing most rapidly, we introduced a cost-cutting policy in the first six months of 2016, which will deliver greater benefits starting in the second half of 2016. We are also continuing our international expansion drive. We moved into new territories and are working hard to raise the visibility of our products in the scientific community, so that the use of single-use instruments for back surgeries gains further traction.”
| in thousands of euros | H1 2016 | H1 2015 | ||
| Adjusted revenue* | 1,204 | 1,045 | ||
| Revenue | 1,303 | 1,307 | ||
| Purchases used and change in inventories | (981) | (894) | ||
| External costs | (1,362) | (1,141) | ||
| Personnel costs | (2,010) | (1,983) | ||
| Other operating expenses | (351) | (308) | ||
| Operating income/(loss) before non-recurring items | (3,402) | (3,019) | ||
| Operating income/(loss) | (3,412) | (3,019) | ||
| Net financial income/(expense) | (158) | 301 | ||
| Net income/(loss) | (3,559) | (2,727) |
Adjusted first-half 2016 revenue up 15%
Following the introduction of the new strategy unveiled by Safe Orthopaedics in March of refocusing on its fastest-growing regions, it recorded adjusted revenue of €1.2 million in the first half of 2016. This represented an increase of 15%, with balanced contributions from France and the Rest of the World. Including the contribution of €0.26 million from the United States in the first half of 2015, total revenue was stable at €1.3 million compared with the year-earlier period.
Safe Orthopaedics has consolidated its sales performance in France and achieved major progress in the Rest of the World.
In parallel, Safe Orthopaedics continued to expand its international distribution network by entering into additional agreements covering Australia, New Zealand and two initial countries in Latin America (Chile and Mexico). Further territories are also expected to be added during the second half of the year.
Launch of a drive to cut operating costs
Safe Orthopaedics’ first-half 2016 operating loss came to €3.4 million, compared with a loss of €3.0 million in the same period of 2015. This increase included €0.4 million in non-recurring expenses incurred as a result of refocusing in France and the Rest of the World and the departure in June of the previous Chief Executive Officer.
After taking into account €0.16 million in net financial expense as a result of currency effects on intragroup cash balances, Safe Orthopaedics recorded a first-half 2016 net loss of €3.6 million, compared with a loss of €2.7 million in the first six months of 2015.
During the period, Safe Orthopaedics launched a cost-cutting policy, which should start to pay off from the second half of 2016.
Stronger cash position
At June 30, 2016, Safe Orthopaedics held €4.3 million in cash. This does not include a €0.5 million subscription by IdInvest Partners in early July following the Shareholders’ Meeting on June 30, 2016 and another €0.5 million subscription raised from IdInvest Partners in the form of OCABSA bonds on July 26, 2016.
Next Press Release: Q3 2016 revenue, October 13, 2016 (after market close)
About Safe Orthopaedics
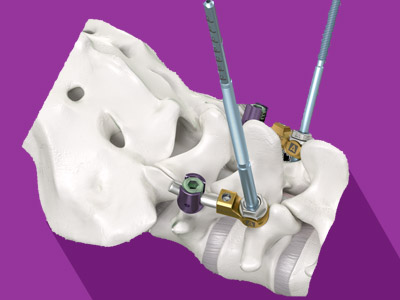
Founded in 2010, Safe Orthopaedics is a French medical technology company that develops and markets an innovative range of sterile implants and associated single-use surgical instruments, with the aim of facilitating safer, optimized and lower-cost spinal surgery. By avoiding the reuse of surgical instruments, Safe Orthopaedics reduces the risk of infection, avoids the cumbersome and unreliable logistics of instrument sterilization, and limits hospital costs. Protected by 17 patent families, the SteriSpine™ Kits are CE-marked and FDA cleared. The company is based at Eragny-sur-Oise (France), and has 34 employees. For more information, visit:www.SafeOrtho.com
* Adjusted for operations in the United States discontinued effective March 1, 2016.
Contacts
Safe Orthopaedics
Thierry Lambert, +33 (0)1 34 21 50 00
CFO
investors@safeorthopaedics.com
or
NewCap
Julien Perez / Valentine Brouchot
Investor Relations
or
Nicolas Merigeau
Media Relations
Tel. : +33 (0)1 44 71 94 94
SafeOrtho@newcap.eu