GAINESVILLE, Fla.–(BUSINESS WIRE)–
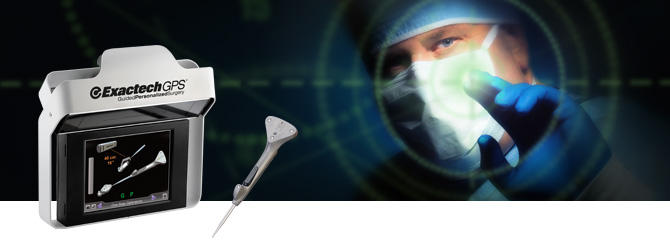
Exactech, Inc. (EXAC), a developer and producer of bone and joint restoration products for hip, knee, shoulder and spine, today announced positive surgeon feedback on first surgeries performed with the ExactechGPS® Guided Personalized Surgery system’s new Total Shoulder Application, which combines preoperative planning with intraoperative computer-assisted technology. Pierre-Henri Flurin, MD, a shoulder specialist at the Clinique du Sport in Bordeaux-Mérignac, France, performed both an anatomic and a reverse total shoulder replacement using Equinoxe® Shoulder System implants and instruments.
This Smart News Release features multimedia. View the full release here:http://www.businesswire.com/news/home/20160929006357/en/
“Our goal is to offer improved accuracy and precision in the placement and orientation of the Equinoxe implants,” said Dr. Flurin. “I was able to plan the case in advance through a virtual simulation and then execute that plan while visualizing the 3-D anatomy in real time, making adjustments based on assessments during surgery. Exactech’s design teams have developed innovative shoulder implants designed to preserve bone and overcome challenges; this new technology is the perfect complement to those implants.”
“In collaboration with our team of engineers located in Florida and France, Dr. Flurin and the rest of the design team brought brilliant expertise to our effort,” said Darin Johnson, Vice President of Marketing, Extremities. “Visualizing the glenoid vault in real time and adjusting the implant orientation to the unique anatomy of the patient should help surgeons improve patient outcomes, which is what we come to work every day to do.”
The shoulder application focuses on guiding the preparation of the glenoid in shoulder arthroplasty. There is wide variation in the position and orientation of glenoid implants because of limited exposure and poor operative landmarks, and studies show that inaccurate placement of the glenoid component has been linked with increased stresses in the implants1, early loosening and poor outcomes.2 The goal of the new ExactechGPS Total Shoulder Application is to allow surgeons to avoid those issues, ultimately providing longer implant survivorship.
Exactech worked closely with a design team of experienced upper extremity specialists to develop this system: Emilie Cheung, MD, Stanford University Department of Orthopaedics (Palo Alto, Calif.); Pierre-Henri Flurin, MD, Clinique du Sport (Bordeaux-Mérignac, France); Richard Jones, MD, Southeastern Sports Medicine (Asheville, N.C.); Moby Parsons, MD, Seacoast Orthopedics and Sports Medicine (Somersworth, N.H.); Paul Saadi, MD, Dallas Bone and Joint Clinic (Dallas, Texas); Thomas Wright, MD, University of Florida Department of Orthopaedics and Rehabilitation (Gainesville, Fla.); and Joseph Zuckerman, MD, NYU Hospital for Joint Diseases (New York, N.Y.).
The ExactechGPS Total Shoulder Application is being piloted in Europe and is currently undergoing premarket review by the FDA.*
About ExactechGPS
ExactechGPS combines surgeon expertise with an advanced computer system to perform the patient’s surgery with a goal of improved accuracy and precision. Personalized for a patient’s unique bone structure and anatomy, ExactechGPS is designed to allow surgeons to decide where to remove bone and place the implant in the optimal position. Exactech first introduced ExactechGPS to surgeons in the United States for knees, and has since broadened its reach by providing medical education and supporting surgeries in more than 11 countries. The technology has steadily gained market acceptance and worldwide reach.
About Exactech
Based in Gainesville, Fla., Exactech develops and markets orthopaedic implant devices, related surgical instruments and biologic materials and services to hospitals and physicians. The company manufactures many of its orthopaedic devices at its Gainesville facility. Exactech’s orthopaedic products are used in the restoration of bones and joints that have deteriorated as a result of injury or diseases such as arthritis. Exactech markets its products in the United States, in addition to more than 30 markets in Europe, Latin America, Asia and the Pacific. Additional information about Exactech can be found at http://www.exac.com.