CAESAREA, ISRAEL (PRWEB) JUNE 30, 2017
OrthoSpace Ltd. (“OrthoSpace” or “the Company”) today announced the publication of positive data on the use of the InSpace™ subacromial spacer for the treatment of massive rotator cuff tears. The case series of 39 shoulders (37 patients) was published in Arthroscopy and demonstrated improved function and reduced pain in patients treated with the InSpace System.
“Patients who fail conservative therapy for massive rotator cuff repair have historically had few options for further treatment of this debilitating, painful condition,” said Julien Deranlot, M.D., principal investigator of the study and an orthopedic surgeon at Clinique Drouot in Paris, France. “The InSpace provides a safe and reliable surgical alternative that can improve shoulder pain and function, allowing patients to have a better quality of life post-operatively.”
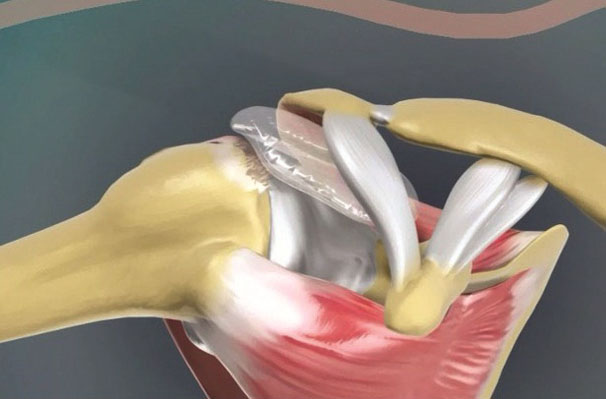
The InSpace System consists of a biodegradable balloon spacer deployed in the subacromial space between the acromion and the humeral head, allowing smooth gliding and frictionless movement between the two bones and emulating the function of the original bursa. It is usually placed arthroscopically in a procedure that requires only 10 minutes to perform.
This study assessed clinical, functional and radiographic outcomes for all patients with symptomatic massive irreparable rotator cuff tears who were treated with the InSpace at the Clinique Drouot between January 2011 and December 2014. Patients included in this study had a minimum of one year and up to three years of follow-up at the time of analysis.
The investigators reported the following results:
- Range of motion was significantly increased for all patients in anterior elevation, abduction and external rotation.
- The mean Constant score, which measures pain, activity level and function, improved from 40 (±14.6) (45 [±15.2] when adjusted for age and gender) at baseline to 59 (±13.7) at one year, and up to 64 (±13.6) (adjusted = 76 [±17.1]) (P < 0.0001) at last follow-up. Notably, there was a significant improvement between the one-year follow-up and three-year follow-up regarding the adjusted Constant score (P=0.02).
- At last follow-up, adjusted Constant score was excellent (greater than 100 points) for three shoulders (9%), good (86-99 points) for eight (23%), fair (65-85 points) for sixteen (45%), and poor (fewer than 65 points) for eight shoulders (23%).
- Among the radiographic outcomes, when assessing for Hamada score (a measure of osteoarthritis), four shoulders progressed by one radiographic stage (15%), and two shoulders progressed by three stages (4%). 32 shoulders had no progression during follow-up.
“We continue to be encouraged by the compelling clinical improvements we see in patients treated with the InSpace System, which are in line with the strong results demonstrated in previous studies,” OrthoSpace CEO Itay Barnea commented. “The system offers a safe, minimally invasive option that can be effectively used to relieve pain and improve shoulder function for patients, especially in elderly patients with chronic pain and disability, poor tissue quality or other health issues, who may not have alternative treatment options.”
The InSpace System is CE Marked in Europe and Israel and is investigational in the U.S. and Canada, where it is currently being evaluated in a prospective, single-blinded, multi-center, randomized, controlled study that will enroll up to 184 patients.
About OrthoSpace Ltd.
OrthoSpace is a privately held medical device company located in Caesarea, Israel. The Company’s product, InSpace, is an orthopedic biodegradable balloon system that is simple, safe and a minimally invasive method that addresses unmet clinical needs in rotator cuff repair. InSpace is CE Marked and commercialized in Europe and Israel and has been granted an Investigational Device Exemption (IDE) to initiate a pivotal human clinical study of the InSpace System in the United States.




![Oska Wellness is Nominated in the 2017 Startup of the Year Competition – West [Pacific – Southern California] 1-2.jpeg](http://www.oasissurg.com/wp-content/uploads/2017/06/1-2-1-880x587.jpeg)