SALT LAKE CITY, April 26, 2018 /PRNewswire/ — DiscGenics, Inc., a clinical stage regenerative medicine company focused on developing cell therapies that alleviate pain and restore function in patients with degenerative diseases of the spine, today announced the first patient has been treated in its phase I/II U.S. clinical trial of IDCT for mild-to-moderate degenerative disc disease (DDD). The treatment took place at Carolina Neurosurgery and Spine Associates in Charlotte, NC, led by the study’s principal investigator, Domagoj Coric, M.D.
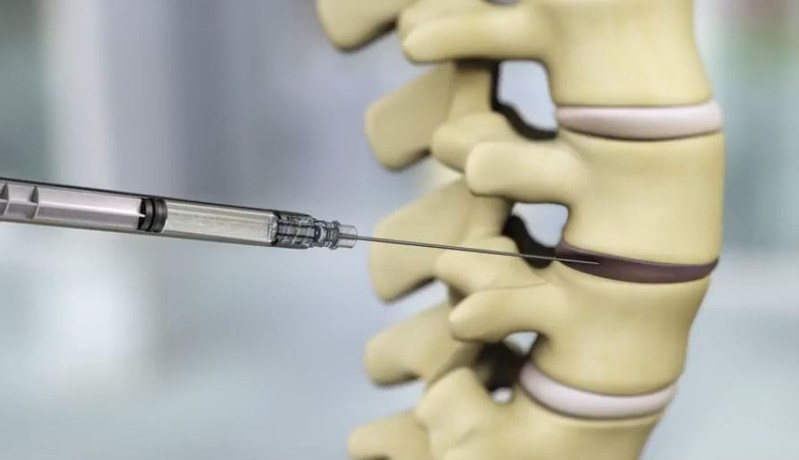
IDCT is an allogeneic (donor-derived), non-invasive cell therapy comprised of proprietary Discogenic Cells and a viscous scaffold carrier. This prospective, randomized, double-blinded, vehicle- and placebo-controlled study is designed to evaluate the safety and preliminary efficacy of IDCT at varying dosage levels in subjects with single-level, symptomatic lumbar DDD, a major cause of chronic low back pain.
“We are thrilled to be participating in the clinical evaluation of this potentially game-changing therapy for patients with DDD,” said Dr. Coric. “If the outcomes observed in human subjects are consistent with preclinical study findings of IDCT, which indicated reduced inflammation and disc height restoration, the result could be reduced pain and disability, offering a truly regenerative therapy for one of the most common causes of chronic low back pain.”
“Commencing clinical evaluation of our first product candidate is a critical milestone for DiscGenics as we continue to advance IDCT as a potentially revolutionary treatment for DDD,” said Flagg Flanagan, Chief Executive Officer and Chairman of the Board of Directors for DiscGenics. “The millions of patients who suffer from low back pain every year currently have limited treatment options, one of which is opioid use for pain management. If proven effective, IDCT could be a vital tool for curtailing opioid addiction among those who suffer from this debilitating condition.”
The trial will take place in up to 10 centers across the U.S. and will enroll approximately 60 subjects. Those who meet all eligibility criteria will be randomized to one of four treatment cohorts: low dose IDCT (n=20), high dose IDCT (n=20), vehicle (n=10) and placebo (n=10).
Each subject will receive a single intradiscal injection of his or her assigned treatment into the target symptomatic lumbar intervertebral disc. Following treatment, subjects will be observed and evaluated for a period of one year, with a one-year extension period.
For more information, please visit clinicaltrials.gov/.
Initiation of this trial was supported by the U.S. Food and Drug Administration’s (FDA) acceptance of the Company’s investigational new drug (IND) application for IDCT, announced in the fourth quarter of 2017.
DiscGenics is one of just two companies in the world with an allogeneic cell-based product for disc degeneration that is pursuing a Biologics License Application (BLA) from the FDA through its rigorous clinical and regulatory IND pathway.
About DiscGenics
DiscGenics is a privately held, clinical stage regenerative medicine company focused on developing cell therapies that alleviate pain and restore function in patients with degenerative diseases of the spine. DiscGenics is harnessing the restorative potential of cells native to the intervertebral disc to develop what we hope will be a profound therapeutic option for millions of patients suffering from the debilitating effects of back pain. DiscGenics’ first product candidate, IDCT, is a homologous, allogeneic injectable cell therapy that utilizes proprietary Discogenic Cells to offer a non-surgical, potentially regenerative solution for the treatment of patients with mild to moderate degenerative disc disease.
SOURCE DiscGenics, Inc.