January 31, 2017 – TORONTO–(BUSINESS WIRE)-
Synaptive Medical Inc., a medical device and technology company, is pleased to announce that North Shore University Hospital, a member of Northwell Health, is the first center on Long Island to acquire the BrightMatter technology, an innovative solution of advanced imaging, planning, navigation, and robotics for complex brain tumor and spinal surgery.
“In our efforts to provide our patients with the absolutely latest technology and expertise, Northwell Health’s Neuroscience Institute and Cancer Institute are delighted to introduce Synaptive’s BrightMatter neurosurgical system to our area,” said Raj K. Narayan, MD, Northwell Health’s senior vice president, neurosurgery service line. “Being one of the first centers in the country to adopt this technology, we will now be able to approach tumors in the brain with visualization of details that will enable us to perform safer procedures and provide better outcomes. The system allows neurosurgeons to use sophisticated magnetic resonance imaging (MRI) data to gain insight into the structure of white matter, which can help them locate neural pathways responsible for a patient’s ability to walk, speak, or see. A very kind gift from the estate of a patient, Mr. Irwin Davis, who himself had a brain tumor several years ago, made this advance possible. We are all very grateful, as will be many future patients.”
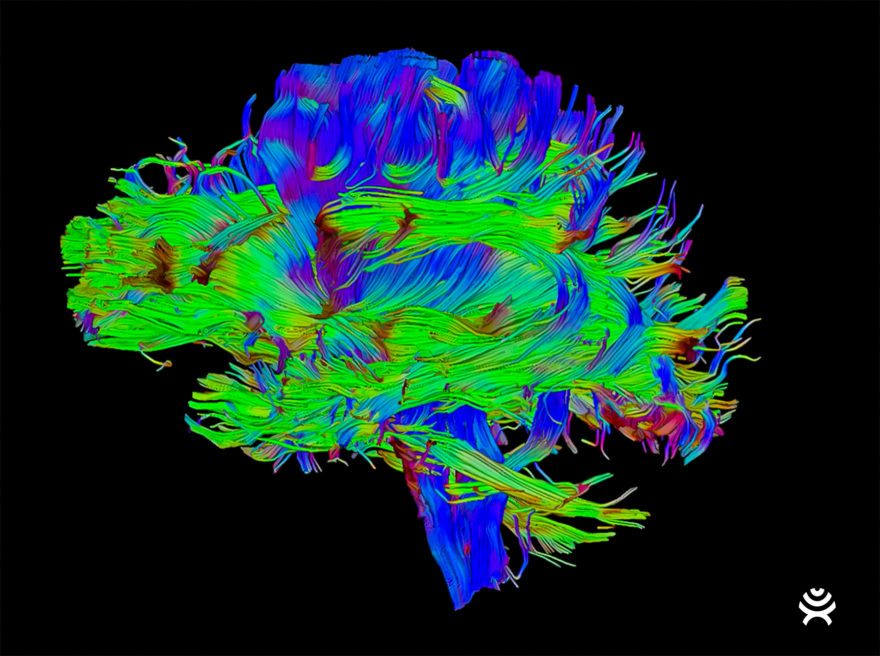
BrightMatter provides neurosurgeons with the latest advancements in visualization tools to perform minimally-invasive, patient-specific procedures. Using an imaging method called diffusion tensor imaging, or DTI, BrightMatter uses tractography to automatically enhance MRI images of the entire brain’s pathways, allowing surgeons to see structures that can’t be seen with the naked eye and consider approaches for optimal navigation. This functionality may allow access to brain locations previously deemed inoperable.
With this information, surgeons can use Synaptive’s high-powered magnification system to view patient anatomy. This optical visualization system, mounted on a robotic arm, follows the surgeon’s tools and shows patient anatomy in unprecedented detail. BrightMatter allows for better surgical ergonomics, facilitates collaboration with operating room staff, and consumes less surgical time without the need to manipulate cumbersome optics.
“With the addition of this state-of-the-art technology, we will not only offer our patients unique treatment options, but also conduct clinical oncology research for discovery of still better approaches as we go forward,” said George Raptis, MD, Northwell Health’s senior vice president, cancer service line. “This is in step with Northwell Health’s ongoing commitment to all the communities we serve.”
Synaptive is uniquely positioned to support Northwell Health’s commitment to be on the forefront of care through state-of-the-art technologies and innovative research. “Collaborating with surgeons to better understand their challenges in the operating room is deeply rooted in Synaptive’s culture,” said Cameron Piron, Synaptive’s president. “Their insights fuel our development work and help our engineering teams to design integrated solutions, both for today and for operating rooms of the future. We look forward to exploring new opportunities with Northwell Health’s neurosurgical team.”
About Northwell Health
Northwell Health is New York State’s largest health care provider and private employer, with 21 hospitals and over 550 outpatient facilities. We care for more than two million people annually in the metro New York area and beyond, thanks to philanthropic support from our communities. Our 61,000 employees – 15,000+ nurses and nearly 3,400 physicians, including nearly 2,700 members of Northwell Health Physician Partners — are working to change health care for the better. We’re making breakthroughs in medicine at the Feinstein Institute. We’re training the next generation of medical professionals at the visionary Hofstra Northwell School of Medicine and the School of Graduate Nursing and Physician Assistant Studies. And we offer health insurance through CareConnect. For information on our more than 100 medical specialties, visit Northwell.edu.
This Smart News Release features multimedia. View the full release here: http://www.businesswire.com/news/home/20170131005866/en/