WALTHAM, Mass., July 31, 2018 (GLOBE NEWSWIRE) — Histogenics Corporation (Histogenics) (Nasdaq:HSGX), a leader in the development of restorative cell therapies (RCTs) that may offer rapid-onset pain relief and restored function, today announced the appointment of E. Lynne Kelley, M.D., FACS as its Chief Medical Officer. Dr. Kelley brings more than 20 years of executive management and surgical experience in medical affairs, clinical operations, regulatory affairs and product development to Histogenics. Dr. Kelley will join Histogenics’ executive team and assume responsibility for leading medical affairs strategy and building out the department in anticipation of NeoCart’s potential commercial launch. Dr. Kelley will also work with the team on preparing the anticipated Biologics License Application (BLA) for NeoCart and spearheading related discussions with the United States Food and Drug Administration (FDA).
“Lynne’s experience as a surgeon, medical affairs executive and educator across a wide variety of therapeutic areas and indications will be critical for Histogenics as we prepare for our top-line data, potential BLA submission and FDA review of NeoCart,” stated Adam Gridley, President and Chief Executive Officer of Histogenics. “We are pleased to have Lynne join us at this exciting time to educate our customers and patients about the benefits of our novel restorative cell therapy platform through training, medical education and collaboration with our future commercial team. In addition, she will be working closely with our team to help drive additional product development initiatives, such as future trials in the U.S. and internationally.”
Dr. Kelley is a board certified general and vascular surgeon having received her medical degree from Dartmouth Medical School and completed her Residency in General Surgery at Dartmouth Hitchcock Medical Center. During her training she was awarded an NIH-sponsored basic science research grant at Harvard Medical School. She completed a Fellowship in Vascular Surgery at Harvard Medical School, Massachusetts General Hospital and was awarded the Marco Polo Fellowship providing advance training in Endovascular Surgery at the University Paris Hospital, Henri Mondor. Dr. Kelley also received a B.A. in Biology from Boston University. Prior to Histogenics, Dr. Kelley held various medical affairs roles within the industry including: Chief Medical Officer at Senseonics, World Wide Vice President of Medical Affairs at Becton Dickinson & Company Medical Surgical Systems Division, Vice President and Medical Director at Kimberly Clark, and Medical Director at Boston Scientific Corporation’s (Boston Scientific) Peripheral Interventions and Vascular Surgery division. Prior to her work at Boston Scientific, Dr. Kelley was an assistant professor of vascular surgery and radiology at Yale University.
“I am thrilled to join the talented, cutting edge team at Histogenics. The potential of NeoCart in restoring function and thus improving the lives of patients with debilitating joint pain is extraordinary,” shared Dr. Kelley. “I look forward to collaborating with the physician community globally to bring this exciting therapy to our patients.”
In connection with the hiring of Dr. Kelley, the Compensation Committee of Histogenics’ Board of Directors approved a grant to Dr. Kelley of a stock option to purchase 200,000 shares of Histogenics’ common stock. The option was granted pursuant to the Nasdaq inducement grant exception as a component of Dr. Kelley’s employment compensation, and was granted as an inducement material to her acceptance of employment with Histogenics in accordance with Nasdaq Listing Rule 5635(c)(4). The option will have an exercise price equal to the closing price of Histogenics’ common stock on July 31, 2018. The option has a ten year term and vests with respect to 25% of the shares of common stock underlying the option on the one year anniversary of Dr. Kelley’s first day of employment with Histogenics and with respect to the remaining shares in equal monthly installments over the following 36 months, subject to Dr. Kelley’s continued service with Histogenics through the applicable vesting dates.
About Histogenics Corporation
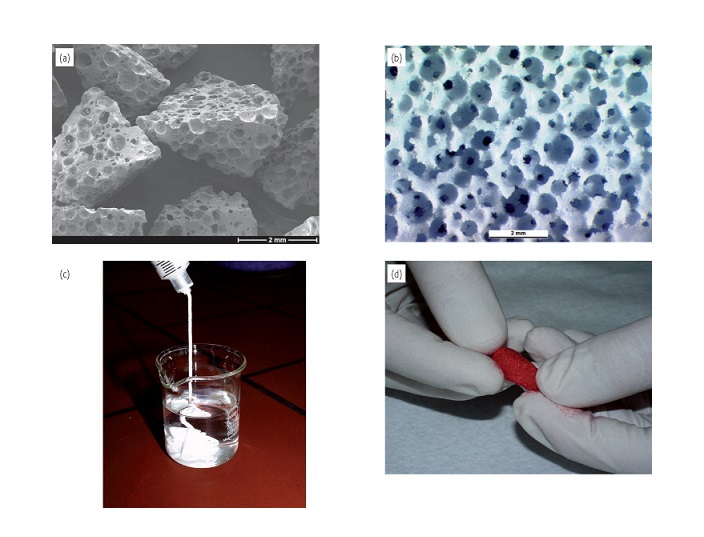
Histogenics (Nasdaq: HSGX) is a leader in the development of restorative cell therapies that may offer rapid-onset pain relief and restored function. Histogenics’ lead investigational product, NeoCart, is designed to rebuild a patient’s own knee cartilage to treat pain at the source and potentially prevent a patient’s progression to osteoarthritis. NeoCart is one of the most rigorously studied restorative cell therapies for orthopedic use. Histogenics completed enrollment of its NeoCart Phase 3 clinical trial in the second quarter of 2017 and expects to report top-line, one-year superiority data in the third quarter of 2018. NeoCart is designed to perform like articular hyaline cartilage at the time of treatment, and as a result, may provide patients with more rapid pain relief and accelerated recovery as compared to the current standard of care. Histogenics’ technology platform has the potential to be used for a broad range of additional restorative cell therapy indications. For more information on Histogenics and NeoCart, please visit www.histogenics.com.
Forward-Looking Statements
Various statements in this release are “forward-looking statements” under the securities laws. Words such as, but not limited to, “anticipate,” “believe,” “can,” “could,” “expect,” “estimate,” “design,” “goal,” “intend,” “may,” “might,” “objective,” “plan,” “predict,” “project,” “target,” “likely,” “should,” “will,” and “would,” or the negative of these terms and similar expressions or words, identify forward-looking statements. Forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions and uncertainties.
Important factors that could cause actual results to differ materially from those reflected in Histogenics’ forward-looking statements include, among others: the timing and success of Histogenics’ NeoCart Phase 3 clinical trial , including, without limitation, possible delays in generating the data from the clinical trial; the ability to obtain and maintain regulatory approval of NeoCart or any product candidates, and the labeling for any approved products; MEDINET’s ability to initiate NeoCart clinical development in Japan in a timely manner; NeoCart’s regulation as a Regenerative Medical Product in Japan; the market size and potential patient population in Japan; the scope, progress, expansion, and costs of developing and commercializing Histogenics’ product candidates; the ability to obtain and maintain regulatory approval regarding the comparability of critical NeoCart raw materials following our technology transfer and manufacturing location transition; the size and growth of the potential markets for Histogenics’ product candidates and the ability to serve those markets; Histogenics’ expectations regarding its expenses and revenue; the sufficiency of Histogenics’ cash resources and the availability of additional financing on commercially reasonable terms; and other factors that are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Histogenics’ Annual Report on Form 10-K for the year ended December 31, 2017 and Quarterly Report on Form 10-Q for the quarter ended March 31, 2018, which are on file with the SEC and available on the SEC’s website at www.sec.gov. Additional factors may be set forth in those sections of Histogenics’ Quarterly Report on Form 10-Q for the quarter ended June 30, 2018, to be filed with the SEC in the third quarter of 2018. In addition to the risks described above and in Histogenics’ Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the SEC, other unknown or unpredictable factors also could affect Histogenics’ results.
There can be no assurance that the actual results or developments anticipated by Histogenics will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Histogenics. Therefore, no assurance can be given that the outcomes stated in such forward-looking statements and estimates will be achieved.
All written and verbal forward-looking statements attributable to Histogenics or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to herein. Histogenics cautions investors not to rely too heavily on the forward-looking statements Histogenics makes or that are made on its behalf. The information in this release is provided only as of the date of this release, and Histogenics undertakes no obligation, and specifically declines any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.