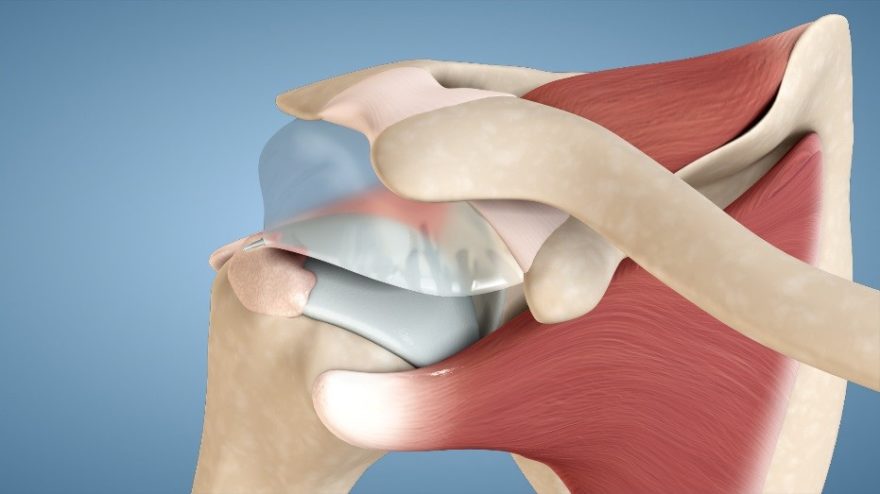
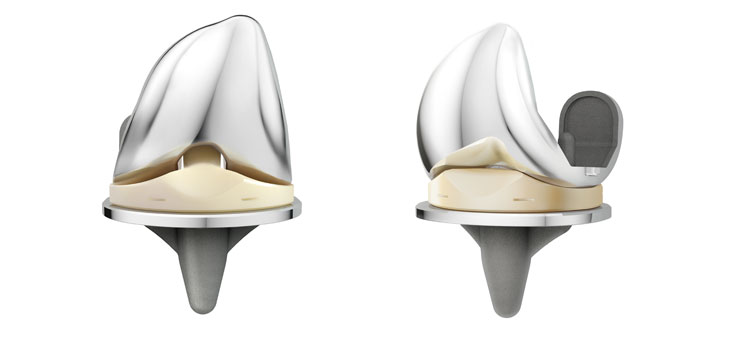
WARSAW, Ind., Dec. 19, 2016 /PRNewswire/ — DePuy Synthes*, part of the Johnson & Johnson Family of Companies, today announced that implant survivorship data from the 2016 Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) confirm positive early results for DePuy Synthes’ ATTUNE® Knee System.1 These data add to other recent registry evidence which has shown that survivorship for the ATTUNE Knee compares favorably to other knee systems in its class.2 In addition, recently-presented interim data have shown improved patient reported outcomes measures with the ATTUNE Knee compared to other leading knee systems.3
Per the 2016 AOANJRR, in which 4,831 ATTUNE Knees are being tracked, the ATTUNE Knee estimated cumulative percent revision was 0.5% (ATTUNE Cruciate Retaining Knee), 0.4% (ATTUNE Posterior Stabilized Knee) at one year.1 This compares favorably to the overall class of cemented total knee arthroplasty (TKA) at one year, which has an estimated cumulative percent revision of 1.0%.1
In addition, interim results from an ongoing study of patients implanted with ATTUNE Posterior Stabilized (PS)TKA Knees showed a reduced incidence of symptomatic crepitus that was half of that compared to non-ATTUNE PS TKA Knees at a minimum of one year .4
“There is a steady cadence of evidence for the ATTUNE Knee that suggests improved outcomes and functional performance3,5, helping patients get back sooner with a reduced hospital length of stay,” said Paul Voorhorst, Vice President of Clinical Research for DePuy Synthes Joint Reconstruction. “We are committed to ongoing monitoring of both the outcomes and economic benefits of this new technology.”
From a health economics standpoint, a large U.S. hospital administrative database review showed ATTUNE Knee patients had a 39% lower odds of discharge to a skilled nursing facility compared to patients who received a TKA with a Triathlon® Knee.6
“These promising early results are encouraging for patients, healthcare providers and payers, and underline the importance of our robust evidence generation program for the ATTUNE Knee,” said Christina Farup, Vice President, Health Economics and Market Access, Johnson & Johnson Medical Devices. “We designed the ATTUNE Knee System with both patients and providers in mind to help health care systems increase patient satisfaction, improve clinical outcomes and reduce overall health care costs.”
The ATTUNE Knee evidence generation program gathers information from many sources, including other joint registries. In addition to the AOANJRR, the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man (NJR) also indicated that the ATTUNE Knee System compares favorably to other cemented total knee systems in its class.2 Per the 2016 NJR, the ATTUNE Knee estimated cumulative percent revision was 1.39% at 3 years (98.61% survivorship) for 4,463 knees, comparing favorably to the class of Cemented TKA that has an estimated cumulative percent revision of 1.50%.2
Advancements in TKA surgery systems, such as the ATTUNE Knee, may not only benefit patients, but the healthcare system as a whole because patients may be able to leave the hospital sooner and return to normal activity. A 2013 study published in The Journal of Bone and Joint Surgery found that societal savings, such as decreased disability costs, return to independent living, and increased work productivity, far exceeded direct costs [of TKA surgery].7
Global thought-leader surgeons, engineers, and experts in fields of study such as kinematics, anthropometrics, polyethylene wear and design collaborated to develop and test the ATTUNE Knee System. Extensive research and science has gone into the design to help improve functional outcomes for patients, performance for surgeons, and efficiency for providers.
About DePuy Synthes
DePuy Synthes, part of the Johnson & Johnson Family of Companies, provides one of the most comprehensive portfolios of orthopaedic solutions in the world. DePuy Synthes solutions, in specialties including joint reconstruction, trauma, neurological, craniomaxillofacial, spinal surgery and sports medicine, are designed to advance patient care while delivering clinical and economic value to health care systems worldwide. For more information, visit www.depuysynthes.com.
*DePuy Synthes represents the products and services of DePuy Orthopaedics, Inc. and its subsidiaries.
The third party trademarks used herein are the trademarks of their respective owners.
© DePuy Synthes 2016. All rights reserved.
References:
|
Australian Orthopedic Association National Joint Replacement Registry 2016 Annual Report
|
|
|
Extracted from Table KT9 Cumulative Percent Revision of Primary Total Knee Replacement with Cemented Fixation
|
|
Femoral
Component
|
Tibial
Component
|
N
Revised
|
N
Total
|
1 Yr
|
3 Yrs
|
5 Yrs
|
7 Yrs
|
10 Yrs
|
15 Yrs
|
|
Attune CR
|
Attune
|
17
|
3199
|
0.5 (0.3, 0.9)
|
|
|
|
|
|
|
Attune PS
|
Attune
|
7
|
1632
|
0.4 (0.2, 0.9)
|
|
|
|
|
|
|
Genesis II CR
|
Genesis II
|
421
|
13019
|
0.9 (0.8, 1.1)
|
2.4 (2.2, 2.7)
|
3.1 (2.8, 3.5)
|
4.0 (3.6, 4.4)
|
4.3 (3.9, 4.7)
|
5.6 (4.7, 6.7)
|
|
Genesis II PS
|
Genesis II
|
518
|
14812
|
1.2 (1.1, 1.4)
|
2.8 (2.6, 3.1)
|
3.7 (3.4, 4.1)
|
4.3 (3.9, 4.7)
|
5.0 (4.5, 5.5)
|
|
|
Journey Oxinium
|
Journey
|
220
|
3032
|
1.4 (1.0, 1.9)
|
4.5 (3.8, 5.3)
|
6.4 (5.5, 7.4)
|
8.8 (7.6, 10.0)
|
|
|
|
Nexgen CR Flex
|
Nexgen
|
254
|
16286
|
0.7 (0.6, 0.8)
|
1.5 (1.3, 1.7)
|
2.0 (1.8, 2.3)
|
2.3 (2.0, 2.6)
|
2.7 (2.2, 3.2)
|
|
|
Nexgen LPS Flex
|
Nexgen
|
838
|
27014
|
0.9 (0.8, 1.0)
|
2.3 (2.1, 2.5)
|
3.2 (3.0, 3.4)
|
3.9 (3.6, 4.2)
|
5.0 (4.7, 5.5)
|
|
|
PFC Sigma CR
|
PFC Sigma
|
277
|
11461
|
0.8 (0.7, 1.0)
|
1.9 (1.6, 2.2)
|
2.4 (2.1, 2.7)
|
2.9 (2.5, 3.3)
|
3.4 (3.0, 3.9)
|
|
|
PFC Sigma PS
|
PFC Sigma
|
241
|
7167
|
1.2 (1.0, 1.5)
|
2.6 (2.2, 3.0)
|
3.2 (2.7, 3.6)
|
3.5 (3.1, 4.0)
|
4.5 (3.9, 5.3)
|
|
|
Triathlon CR
|
Triathlon
|
497
|
25632
|
0.8 (0.7, 0.9)
|
2.1 (1.9, 2.3)
|
2.6 (2.4, 2.8)
|
3.0 (2.7, 3.4)
|
3.8 (3.2, 4.5)
|
|
|
Triathlon PS
|
Triathlon
|
185
|
5886
|
1.5 (1.2, 1.8)
|
3.2 (2.7, 3.7)
|
4.0 (3.4, 4.6)
|
4.6 (3.9, 5.3)
|
|
|
|
Vanguard CR
|
Maxim
|
133
|
6778
|
0.6 (0.4, 0.8)
|
2.2 (1.8, 2.7)
|
2.8 (2.4, 3.4)
|
3.3 (2.7, 4.0)
|
|
|
|
Vanguard PS
|
Maxim
|
166
|
3500
|
1.9 (1.5, 2.4)
|
4.6 (3.9, 5.4)
|
5.7 (4.9, 6.7)
|
6.8 (5.7, 8.0)
|
|
|
|
Table KT22 Cumulative Percent Revision of Primary Total Knee Replacement by Fixation (Primary Diagnosis OA)
|
|
Fixation
|
N
Revised
|
N
Total
|
1 Yr
|
3 Yrs
|
5 Yrs
|
7 Yrs
|
10 Yrs
|
15 Yrs
|
|
Cemented
|
8439
|
258789
|
1.0 (0.9, 1.0)
|
2.6 (2.5, 2.6)
|
3.4 (3.4, 3.5)
|
4.2 (4.1, 4.3)
|
5.1 (5.0, 5.3)
|
7.3 (6.9, 7.7)
|
|
Cementless
|
4612
|
103903
|
1.2 (1.1, 1.3)
|
3.2 (3.1, 3.3)
|
4.3 (4.2, 4.4)
|
5.0 (4.9, 5.2)
|
6.1 (5.9, 6.3)
|
8.1 (7.7, 8.6)
|
|
Hybrid
|
3964
|
119262
|
0.9 (0.9, 1.0)
|
2.5 (2.4, 2.6)
|
3.3 (3.2, 3.5)
|
3.9 (3.8, 4.1)
|
4.8 (4.7, 5.0)
|
6.6 (6.2, 7.0)
|
|
TOTAL
|
17015
|
481954
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Note: Excluding cementless Genesis Oximium and Profix Oxinium femoral prostheses
|
|
2.
|
National Joint Registry for England, Wales, Northern Ireland and the Isle of Man Annual Report. (2016). Tables 3.28 and 3.24 (a). Retrieved from:http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/13th%20Annual%20Report/07950%20NJR%20Annual%20Report%202016%20ONLINE%20REPORT.pdf
|
|
3.
|
Hamilton, W., Himden, S., Brenkel, I., Clatworthy, M., Dwyer, K., Lesko, J. and Kantor, S. Early Patient Reported Outcomes With New Primary vs. Contemporary Total Knee Arthroplasty: A Comparison of Two Worldwide, Multi-Center Prospective Studies. International Society for Technology in Arthroplasty (ISTA): e-Poster, 5-8 October 2016, Boston, MA. Based on interim data. The leading knee systems included: 89% P.F.C.® SIGMA®, 3% Zimmer NexGen®, 7% Stryker Triathlon®, 1% Other.
|
|
4.
|
Toomey, S., Daccach, J., Shah, J., Himden, S., Lesko, J. and Hamilton, W. Comparing the Incidence of Patellofemoral Complications in a New Total Knee Arthroplasty (TKA) System vs. Currently Available Products in Two, Worldwide, Multi-Center, Prospective Clinical Studies. While not statistically significant, the trend is promising and follow-up is ongoing. Based on interim data.
|
|
5.
|
Clatworthy, M. (2015). An Early Outcome Study of the ATTUNE® Knee System vs. the SIGMA® CR150 Knee System. DePuy Synthes Companies White Paper. DSUS/JRC/0814/0418(1). In an IRB approved early outcomes study, physiotherapists collected data on 40 patients implanted with ATTUNE Knees and 40 patients with SIGMA® CR150 Knees. The results demonstrated that patients implanted with the ATTUNE Knee had statistically significant improvements in some early outcomes, other outcomes demonstrated a trend favoring the ATTUNE Knee, and some outcomes were equivalent.
|
|
6.
|
Etter, K., Lerner, J., de Moor, C, Yoo, A., Kalsekar, I. (2016). PMD10-Comparative Effectiveness of ATTUNE® Versus Triathlon® Total Knee Systems: Real-World Length of Stay and Discharge Status.” Value in Health 19(3): A298. Premier Perspective™ Database analysis including 38 hospitals, representing 1,178 primary, unilateral TKAs with the ATTUNE® Knee and 5,707 primary, unilateral TKAs with Triathlon™. The analysis found that the patients implanted with the ATTUNE Knee had statistically shorter length of stay and were more frequently discharged home vs. a skilled nursing facility compared to the TKAs with Triathlon®.
|
|
7.
|
Ruiz D, Koenig L, Dall T, et al. The Direct and Indirect Costs to Society of Treatment for End-Stage Knee Osteoarthritis. J Bone Joint Surg Am., 2013; 95: 1473-80.
|
DSUS/JRC/1216/1887
SOURCE DePuy Synthes