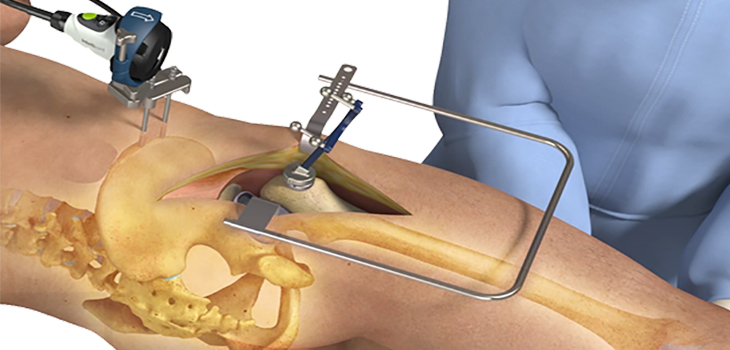
WATERLOO, ON, March 7, 2017 /PRNewswire/ – Intellijoint Surgical® Inc., a privately-held Canadian medical technology company, announces FDA clearance of intellijoint HIP® Anterior, the newest application to the intellijoint HIP suite. intellijoint HIP Anterior is a 3D mini-optical navigation solution that provides quantifiable, intraoperative measurements for cup position, leg length, and offset to orthopaedic surgeons performing Direct Anterior Approach (DAA) Total Hip Arthroplasty (THA).
“intellijoint HIP Anterior has been developed by a group of brilliant engineers with deep understanding of the steps involved in direct anterior hip replacement, which allows surgeons to integrate the device easily into their workflow,” said Dr. Javad Parvizi, member of Intellijoint Surgical’s Scientific Advisory Board and DAA orthopaedic specialist at Thomas Jefferson University Hospital’s Department of Orthopedic Surgery. “intellijoint HIP Anterior provides quantifiable, intraoperative measurements that allows surgeons to optimally position the components, restore limb length and offset. intellijoint HIP Anterior stands to eliminate fluoroscopy verification for cup position, leg length and offset that is often used by surgeons.”
Per bench top validation testing cleared by the FDA, 20 separate simulations were performed with intellijoint HIP Anterior accurately measuring anteversion to within 0.47°and inclination to within 0.65° when compared to radiographic scans. intellijoint HIP Anterior was intuitively designed to provide cup position measurements in relation to the supine coronal plane or Anterior Pelvic Plane, depending on surgeon’s preference.
“The low-cost fee-per-case pricing model of intellijoint HIP Anterior allows me to utilize navigation for all of my total hip replacements with the Direct Anterior Approach.” commented Dr. Michael Bradley, President/CEO of Orthopedics Rhode Island. “My DAA patients have peace of mind knowing that their hip implants are positioned exactly how I planned pre-operatively, thanks to intraoperative measurements from intellijoint HIP Anterior.”
Intellijoint Surgical will be showcasing the full intellijoint HIP suite including intellijoint HIP Anterior at AAOS 2017 Annual Meeting – Booth #5551 from March 15 – 18, 2017 in San Diego, California, USA. intellijoint HIP Anterior is currently in Limited Market Release and will be available in New York, Illinois, Rhode Island and Houston, Texas in early summer 2017.
About Intellijoint Surgical:
Intellijoint Surgical® develops and commercializes surgical navigation solutions. Intellijoint’s flagship product, intellijoint HIP® provides surgeons with real-time, intraoperative measurements to ensure proper positioning of orthopaedic implants during Total Hip Arthroplasty. Intellijoint is committed to driving clinical results through the development of solutions that are accessible, fast, and easy-to-use. Guided by a scientific advisory board comprised of Dr. Allan Gross, an orthopaedic surgeon at Mount Sinai Hospital, and members, Dr. Javad Parvizi at Thomas Jefferson University Hospital, Dr. Michael Cross at Hospital for Special Surgery, Dr. Wayne Paprosky at Rush University Medical Center, and Dr. Ran Schwarzkopf at NYU School of Medicine, Intellijoint is setting the new standard in miniature 3D surgical navigation.
Intellijoint Surgical is the recipient of the 2015 North American Frost & Sullivan Enabling Technology Leadership Award and the Futurpreneur Shopify True Grit Award 2016.
For more information, please visit: www.intellijointsurgical.com
SOURCE Intellijoint Surgical Inc.