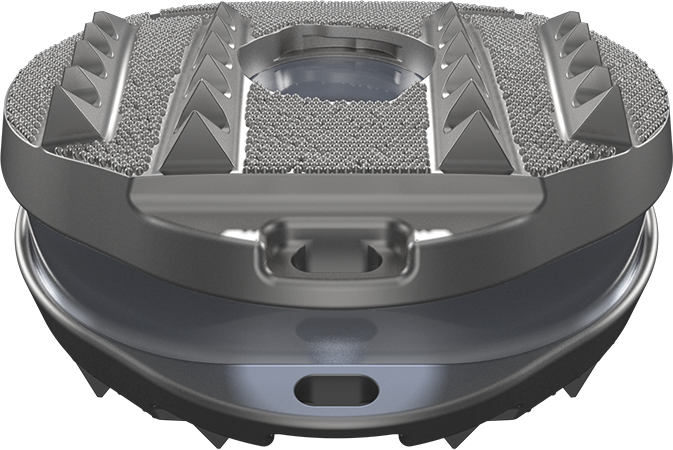
DUBLIN – September 18, 2017 – Medtronic plc (NYSE:MDT) today announced FDA approval and U.S. launch of the Intellis(TM) platform for the management of certain types of chronic intractable pain. The Intellis platform was designed to overcome limitations with current spinal cord stimulation (SCS) systems, such as battery performance, and can power the EvolveSM workflow*, which standardizes guidance and balances high-dose (HD) and low-dose (LD) therapy settings. The Intellis platform can record and track patient activity 24/7 and is managed on the Samsung Galaxy Tab S2 tablet interface, enabling physicians to address the subjective and personal nature of chronic pain by monitoring progress and making modifications to better suit their patients’ therapy needs.
Duke University Medical Center in Durham, N.C. implanted one of the first patients in the U.S. with the Intellis device.
“Chronic pain is challenging to manage. Having real-time data can provide more information about patients’ quality of life changes,” said Dr. Lance Roy, pain medicine specialist at Duke University Medical Center. “This platform represents a welcome new option for managing some kinds of chronic pain. New non-opioid treatment options are important given the national crisis related to opioid abuse.”
Back problems are one of the top 10 most expensive medical conditions, with an estimated 30 percent of the 300,000 patients annually that undergo lumbosacral spine procedures developing chronic intractable pain.1 Chronic pain can negatively impact all aspects of a person’s life – relationships, work productivity and activities of daily living, yet it remains under-recognized and undertreated.1 Neurostimulation has been proven to provide effective long-term pain relief and improve quality of life, in addition to being a treatment option for patients interested in trying a non-drug alternative.2-6
“Drawing upon our 40-year legacy in SCS, the launch of the Intellis platform isn’t just about a new device, but about combining cutting edge hardware with optimal therapy through the Evolve workflow to enable personalized, long-term pain relief,” said Marshall Stanton, M.D., senior vice president and president of Medtronic’s Pain Therapies division, which is part of the Restorative Therapies Group. “Medtronic is committed to addressing patient needs, so the Intellis platform was designed based on what is most important to patients and physicians. We considered the entire patient journey – starting with the primary goal of optimal pain relief and access to important diagnostic tools, like MRI, to ease of use with simplified programming, faster recharge and a smaller implant.”
About the Intellis(TM) Platform
The Intellis platform can help optimize treatment and improve patient-physician communication by tracking and sharing daily activities, body positions and therapy usage and by giving physicians an objective look at mobility and progress. The Intellis platform also addresses a common patient complaint: battery recharge issues. With Medtronic’s proprietary Overdrive(TM) battery technology, the Intellis battery can be fully recharged from empty to full in approximately one hour and physicians can now estimate recharge intervals based on therapy settings.
Additional advances in the Intellis platform include secure wireless Samsung Galaxy Tab S2 programmers for physicians that enable faster delivery of evolving workflows and software upgrades. The Intellis implantable neurostimulator was designed for improved patient comfort and is the world’s smallest fully implantable SCS neurostimulator. The Intellis platform also includes both Medtronic’s proprietary SureScan(TM) MRI technology for the broadest access available to MRI diagnostic imaging and simple eligibility determination, which allows MRI scans anywhere on the body under certain conditions, as well as AdaptiveStim(TM) technology for automatic adjustments to deliver the right therapy dose to the right location, as the pain target shifts based on body position.
“We are excited to partner with Medtronic in their aim to simplify programming, and streamline therapy management with the Intellis platform,” said Dr. Dave Rhew, chief medical officer and head of Healthcare and Fitness for Samsung Electronics America. “Samsung’s Galaxy tablets-secured by the HIPAA-ready Samsung Knox mobile security platform-will support future Medtronic therapies and over the air (OTA) software upgrades to ensure clinicians using Intellis have access to the most up-to-date solutions.”
About Spinal Cord Stimulation
Medtronic neurostimulation therapy for chronic intractable pain uses a medical device placed under a patient’s skin to deliver mild electrical impulses through a lead implanted in the epidural space to block pain signals from going to the brain. SCS is a non-opioid therapy that is clinically proven and cost-effective for treating chronic pain. Multiple randomized controlled trials have demonstrated that SCS provides more effective pain relief than both re-operation and conventional medical management.2-4, 7
|
| Medtonic’s Intellis(TM) Spinal Cord Stimulation Platform |
| Click the thumbnail above for a larger image. |
About Medtronic Pain Therapies
Medtronic has the broadest portfolio of pain therapies, which have been in use for over 40 years and have benefited hundreds of thousands of patients worldwide. Medtronic developed and leads the field of neuromodulation, the targeted and regulated delivery of electrical pulses and pharmaceuticals to specific sites in the nervous system, and continues to innovate and bring patient-centric advances.
About Medtronic
Medtronic plc (www.medtronic.com), headquartered in Dublin, Ireland, is among the world’s largest medical technology, services and solutions companies – alleviating pain, restoring health and extending life for millions of people around the world. Medtronic employs more than 84,000 people worldwide, serving physicians, hospitals and patients in approximately 160 countries. The company is focused on collaborating with stakeholders around the world to take healthcare Further, Together.
All other brands, product names, company names, trademarks and service marks are the properties of their respective owners. All rights reserved.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic’s periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
*A workflow is guidance only and physicians should use their medical judgement and product labeling to optimize therapy for individual patients, which may require discontinuation or modification of a workflow.
References:
1. Mekhail N, Wentzel DL, Freeman R, Quadri H. Counting the costs: case management implications of spinal cord stimulation treatment for failed back surgery syndrome. Prof Case Manag. 2011;16(1):27-36.
2. North RB., Kidd DH., Farrokhi F, et al. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurg; 56: 98-106 (2005).
3. Kumar K., Taylor RS., Jacques L, et al., Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicenter randomised controlled trial in patients with failed back surgery syndrome. Pain; 132: 179-188. (2007).
4. Kemler MA., De Vet HCW., Barendse GAM et al., The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trial. Ann Neurol; 55: 13-18 (2004).
5. Taylor RS, Spinal cord stimulation in Complex Regional Pain Syndrome and Refractory Neuropathic Back and Leg Pain/Failed Back Surgery Syndrome: results of a systematic review and meta-analysis. J Pain Symptom Manage; 31: S13-S19 (2006).
6. Cameron T, Safety and efficacy of spinal cord stimulation for the treatment of chronic pain – a 20 year literature review. J Neurosurg Spine; 100: 254-267 (2004).
7. Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63(4):762-70.
Medtronic Photo
Contacts:
Sara Thatcher
Public Relations
+1-901-399-2098
Ryan Weispfenning
Investor Relations
+1-763-505-4626