October 25, 2017
LEWISVILLE, Texas–(BUSINESS WIRE)–Orthofix International N.V. (NASDAQ:OFIX), a diversified, global medical device company, today announced that its STIM onTrack™ mobile app has earned the 2017 Spine Technology Award in the Cervical Care category from Orthopedics This Week, the most widely read publication in the Orthopedics industry. The award will be presented today at the North American Spine Society (NASS) annual meeting in Orlando, FL. and published in upcoming issues of Orthopedics This Week and Orthopedics This Month Spine.
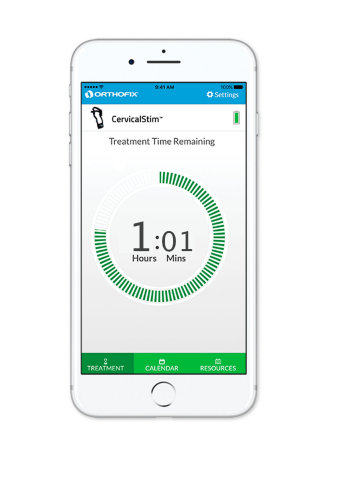
The STIM onTrack technology for mobile devices was introduced in 2017 and works with the latest generation Orthofix CervicalStim™ and SpinalStim™ bone growth stimulators. The mobile app includes a first-to-market feature that enables physicians to receive real-time data on how their patients are adhering to their prescribed treatment, allowing them to adjust and personalize follow-up protocols to help achieve better outcomes. In parallel, the app provides patients with a daily treatment reminder and a device usage calendar to help them adhere to their prescription and take an active role in their spinal fusion recovery. The STIM onTrack app is free and available through the iTunes App Store.
The STIM onTrack technology is one of 10 medical device advancements being recognized by Orthopedics This Week that represent significant advancements in their respective categories.
“The Spine Technology Awards are intended to bring increased recognition to exemplary and innovative spine surgery products and the engineering teams and inventors who create them,” said Robin Young, Founder and Publisher of Orthopedics This Week and RRY Publications. “We are pleased to present Orthofix with this award in recognition of the STIM onTrack mobile app and its potential to facilitate therapy compliance and ultimately improve patient outcomes.”
“We are honored to accept this award on behalf of our team of dedicated people who work hard every day to develop innovative technologies like the STIM onTrack app,” said Brad Niemann, President of the Orthofix BioStim strategic business unit. “This new mobile application and our latest generation CervicalStim and SpinalStim bone growth stimulators are great examples of the merger between our products and technology advancements that are designed to improve patient outcomes. We are proud to partner with physicians to help patients redefine their recovery process through better tracking and adherence to their therapy.”
About Bone Growth Stimulators
Orthofix announced the U.S. Food and Drug Administration (FDA) and European CE Mark approval of its next-generation CervicalStim and SpinalStim bone growth stimulators in January 2017. The U.S. devices feature the STIM onTrack mobile app. These Class III medical devices use a low-level pulsed electromagnetic field (PEMF) designed to activate cellular pathways that stimulate the proliferation and differentiation of mesenchymal stem cells to augment the body’s natural healing process, providing patients with a safe, noninvasive treatment option for promoting post-operative lumbar and cervical fusion.
The SpinalStim device is the only bone growth therapy device approved by the FDA as both a lumbar spinal fusion adjunct to improve fusion outcomes and as a non-surgical treatment for spinal pseudoarthrosis. Additionally, the CervicalStim device is the only bone growth therapy device approved by the FDA as a noninvasive, adjunctive treatment option for improving cervical fusion outcomes in high risk patients. Together, these devices are the number one prescribed bone growth stimulators for spinal fusion.
In October 2016, The North American Spine Society (NASS) issued first-of-its-kind coverage recommendations for electrical bone growth stimulators. These evidence-based coverage policy recommendations support the use of PEMF stimulation devices as an adjunct to spinal fusion surgery.
Orthofix invites those attending the NASS Annual Meeting to visit Booth #1500, Hall WE1 to learn more about the STIM onTrack mobile app and our next-generation bone growth stimulation devices.
About Orthopedics This Week
Orthopedics This Week, a four time winner of the MORE awards for journalistic excellence, is dedicated to delivering breaking news, analysis and commentary about the Orthopedics industry. Published 40 times a year, Orthopedics This Week covers what matters most in healthcare through the distribution of unique and valuable information to professionals who get up every day and work in the orthopedic community.
About Orthofix
Orthofix International N.V. is a diversified, global medical device company focused on improving patients’ lives by providing superior reconstructive and regenerative orthopedic and spine solutions to physicians worldwide. Headquartered in Lewisville, TX, the company has four strategic business units that include BioStim, Biologics, Extremity Fixation and Spine Fixation. Orthofix products are widely distributed via the company’s sales representatives, distributors and subsidiaries. In addition, Orthofix is collaborating on research and development activities with leading clinical organizations such as Brown University, Sinai Hospital of Baltimore, Cleveland Clinic, Texas Scottish Rite Hospital for Children and MTF Biologics. For more information, please visit www.orthofix.com.
Forward-Looking Statements
This communication contains certain forward-looking statements under the Private Securities Litigation Reform Act of 1995. These forward-looking statements, which may include, but are not limited to, statements concerning the projections, financial condition, results of operations and businesses of Orthofix and its subsidiaries, are based on management’s current expectations and estimates and involve risks and uncertainties that could cause actual results or outcomes to differ materially from those contemplated by the forward-looking statements. The forward-looking statements in this release do not constitute guarantees or promises of future performance. Factors that could cause or contribute to such differences may include, but are not limited to, risks relating to: practices of health insurance companies and other third-party payors with respect to reimbursement for our PEMF devices and other risks described in the “Risk Factors” section of our Annual Report on Form 10-K for the fiscal year ended December 31, 2016, as well as in other reports that we file in the future. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. The Company undertakes no obligation to update or revise the information contained in this press release.
Contacts
Orthofix International N.V.
Mark Quick, 214-937-2924
Investor Relations
markquick@orthofix.com
or
Denise Landry, 214-937-2529
Media Relations
deniselandry@orthofix.com