SAN DIEGO, July 9, 2018 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally-integrated solutions, today announced that the first peer-reviewed publication from the NuVasive-sponsored multicenter Integrated Global Alignment (iGA®) study is now available in the journal Spine. This initial study evaluated the preoperative prevalence and incidence of postoperative spinopelvic malalignment in patients who underwent one- or two-level lumbar fusions for degenerative (non-deformity) indications.
Spinopelvic malalignment (i.e., Pelvic Incidence (PI) minus Lumbar Lordosis (LL) greater than or equal to 10°) following lumbar fusion has been shown to be associated with lower postoperative health-related quality of life and elevated risk of adjacent segment failure; however, the incidence of this in short-segment degenerative lumbar fusions from a large sample of patients had been previously unreported.1
The article, “A multicenter radiographic evaluation of the rates of preoperative and postoperative malalignment in degenerative spinal fusions,” reports on the rates of malalignment in nearly 600 patients whose measurements were retrospectively acquired. Researchers measured lateral preoperative and postoperative lumbar radiographs of one- or two-level lumbar fusion patients using the NuVasive iGA platform. The study was conducted at 18 institutions with 24 treating investigators in the United States. Patients were grouped as either aligned (PI-LL<10°) or malaligned (PI-LL≥10°) both pre- and postoperatively.
The results show malalignment is common both before and after short-segment degenerative fusions. Preoperatively, 173 (30 percent) patients undergoing one- or two-level lumbar fusion surgery for degenerative conditions exhibited malalignment and postoperatively, 161 (28 percent) patients were malaligned. These relatively high rates of pre- and postoperative malalignment, even in degenerative cases, demonstrate this is not a deformity-only problem and alignment should be measured in all cases.
“This study further demonstrates that incorporating alignment measurements using iGA into our surgical planning directly translates to our patients and the clinical benefits/outcomes we know are influenced by achieving global spinal alignment,” said Jean-Christophe Leveque, M.D., from the Neuroscience Institute, Virginia Mason Hospital and Medical Center in Seattle. “Alignment in deformity procedures has been relevant for years, but this is the first multicenter study to show just how common malalignment is both before and after short-segment, degenerative fusions. We need to shift our mindset as spine surgeons, recognize these results from simple degenerative cases and start measuring spinopelvic parameters in all of our lumbar fusion cases, especially those that involve L4 through S1.”
The conclusion of the study was that alignment preservation and restoration considerations should be incorporated into the decision-making of all lumbar spinal fusions. Prior to this research, spinal alignment considerations were only thought necessary for spinal deformity cases.
The published results of this study and two additional iGA studies evaluating 1) changes between preoperative standing and intraoperative recumbent alignment and 2) the role of pre- and intraoperative alignment planning on surgical execution and outcomes, will be on the podium at the North American Spine Society (NASS) Annual Meeting in September.
“NuVasive has been focused on the importance of alignment and the impact it has on achieving surgical goals and enhanced clinical outcomes well before we launched iGA in 2015,” said Matt Link, executive vice president, strategy, technology, and corporate development. “We have partnered with some of the largest academic institutions in the country to lead the research efforts and build the scientific evidence to advance the understanding of spinal alignment and the importance it plays in surgical decision-making—further transforming spine and how surgeons approach treatment. These studies are helping drive the future of iGA and adding further value and credibility to our systems-based spine solutions.”
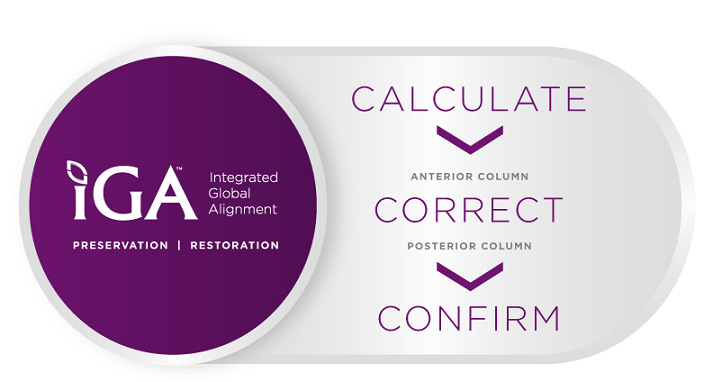
Integrated Global Alignment—Why Alignment Matters™
NuVasive is committed to a global approach for assessing, preserving, and restoring spinal alignment in an effort to promote surgical efficiencies, lasting patient outcomes, and improved quality of life. Integration across the surgical workflow allows the surgeon to confidently and reproducibly calculate, correct and confirm optimal spinal alignment.
To learn more about the NuVasive Integrated Global Alignment platform, visit https://www.nuvasive.com/solutions/iga-integrated-global-alignment/.
About NuVasive
NuVasive, Inc. (NASDAQ: NUVA) is the leader in spine technology innovation, focused on transforming spine surgery and beyond with minimally disruptive, procedurally-integrated solutions designed to deliver reproducible and clinically-proven surgical outcomes. The Company’s portfolio includes access instruments, implantable hardware, biologics, software systems for surgical planning, navigation and imaging solutions, magnetically adjustable implant systems for spine and orthopedics, and intraoperative monitoring service offerings. With over $1 billion in revenues, NuVasive has an approximate 2,400 person workforce in more than 40 countries serving surgeons, hospitals and patients. For more information, please visit www.nuvasive.com.
Forward-Looking Statements
NuVasive cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that involve risks, uncertainties, assumptions and other factors which, if they do not materialize or prove correct, could cause NuVasive’s results to differ materially from historical results or those expressed or implied by such forward-looking statements. The potential risks and uncertainties which contribute to the uncertain nature of these statements include, among others, risks associated with acceptance of the Company’s surgical products and procedures by spine surgeons, development and acceptance of new products or product enhancements, clinical and statistical verification of the benefits achieved via the use of NuVasive’s products (including the iGA platform), the Company’s ability to effectually manage inventory as it continues to release new products, its ability to recruit and retain management and key personnel, and the other risks and uncertainties described in NuVasive’s news releases and periodic filings with the Securities and Exchange Commission. NuVasive’s public filings with the Securities and Exchange Commission are available at www.sec.gov. NuVasive assumes no obligation to update any forward-looking statement to reflect events or circumstances arising after the date on which it was made.
1Leveque JA, Segebarth B, Schroerlucke SR, et al. A multicenter radiographic evaluation of the rates of preoperative and postoperative malalignment in degenerative spinal fusions. Spine 2018;43(13):E782-89.
SOURCE NuVasive, Inc.