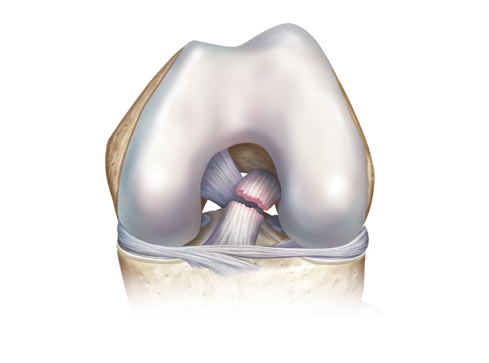
ROSEMONT, Ill., Aug. 21, 2018 /PRNewswire/ — One of the most common knee injuries is an anterior cruciate ligament (ACL) sprain or tear, and doctors are seeing a greater number of older athletes end up in their offices with the injury. An estimated 200,000 ACL-related injuries occur annually in the United States.
Some patients choose to undergo surgical reconstruction of the ACL while others opt for non-surgical intervention. A new review article published in the August 15th issue of the Journal of the American Academy of Orthopaedic Surgeons (JAAOS) discusses surgical and non-surgical treatment options for patients over the age 40 with ACL injuries and proposes shared decision-making about care between patients and doctors.
“The benefits of staying active throughout your life greatly outweigh the risks,” emphasized lead study author and orthopaedic surgeon Matthew Salzler, MD. “ACL tears are an unfortunate but treatable injury that can occur, especially in sports that require sudden twisting and pivoting movements.”
Aging ACLs have a reduced potential to heal and a lower capacity to bear significant strain, as demonstrated in one study highlighted in the review article that compared rats injected with younger and older human ACL-derived cells. Older ACLs may also show greater degeneration.
The review article authors highlight that deciding between surgical and non-surgical treatment options depends on a patient’s activity level and should be a part of a doctor’s initial evaluation when treating patients for an ACL injury. The patient’s history of knee injuries and functional needs should be considered.
According to the article patients of all ages benefit from the rest, ice, compression and elevation (RICE) approach; therefore, this should be the initial management for ACL injuries. This helps reduce pain and swelling, and protects the knee from additional injury. In addition, early physical therapy may help return range of motion and knee stability.
The review article also highlights that the “rule of thirds” has been used for over 25 years to describe different types of patients. They may be classified as:
- Copers: Able to resume all of their previous activity without any issues for at least one year after the injury.
- Adapters: Modify or reduce activity after their injury.
- Non-copers: Require ACL reconstructive surgery because they can’t get through everyday tasks.
The authors explained that though this pattern is not evidence-based, the concepts are relevant when treating patients over 40 as they’re more likely than younger patients to modify their activity over opting for surgical reconstruction.
The article states that the ideal candidates for reconstructive surgery are generally patients who haven’t shown enough improvement with physical therapy alone, want to participate in sports that require sudden pivoting or cutting – like basketball or skiing – and don’t have extensive degenerative changes.
In two systematic reviews of older patients who underwent ACL reconstruction, improvements and positive outcomes were found in knee stability and patient satisfaction. The Swedish National Anterior Cruciate Ligament Register also reported that while older patients had worse knee injury and osteoarthritis scores before surgery than younger patients, their scores after surgery were markedly better, and they showed the greatest improvement one, two, and five years after surgery.
“The outcomes of ACL reconstruction surgeries in patients over 40 are as good as – if not better than – those in younger patients,” says Dr. Salzler.
For patients leaning towards avoiding surgery, the study authors point out that there is conflicting data on the outcomes of nonsurgical treatment in those older than 40. One study of patients with ACL injuries aged 40 to 59 years that did not have surgery had worse outcomes than those that underwent reconstruction. However, in a separate study of Alpine skiers, those treated without surgery fared better than surgical patients.
“As with younger patients, an insufficient or torn ACL is likely to lead to an increased risk of knee instability, meniscal tears, and arthritis,” Dr. Salzler explains. “Whether a patient undergoes surgery or attempts non-operative management of an ACL tear requires a discussion with their orthopaedist.”
The full study is available at: http://bit.ly/2mq18yI
More information about the AAOS and JAAOS
Follow the AAOS on Facebook, Twitter and Instagram
Follow the conversation about JAAOS on Twitter
Disclosures
From Department of Orthopaedics, Tufts Medical Center, Boston, MA (Dr. Salzler), the Department of Orthopedics, University of Colorado at Denver, Anschutz Medical Campus, Aurora, CO (Dr. Chang), and Boston Sports and Shoulder Center, New England Baptist Hospital, Waltham, MA (Dr. Richmond). Dr. Salzler or an immediate family member is a board member or committee member of the American Orthopaedic Society for Sports Medicine. Dr. Richmond or an immediate family member serves as a paid consultant to Histogenics, Mitek, and Visgo Therapeutics; has received royalties and financial or material support from Springer and Wolters Kluwer Health–Lippincott Williams & Wilkins; and is a board member or committee member of the Arthroscopy Association of North America and Eastern Orthopaedic Education Foundation. Neither Dr. Chang nor any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article. J Am Acad Orthop Surg 2018; 0:1-9 DOI: 10.5435/JAAOS-D-16-00730 Copyright 2018 by the American Academy of Orthopaedic Surgeons.
SOURCE American Academy of Orthopaedic Surgeons
Related Links
http://www.aaos.org