DUBLIN–(BUSINESS WIRE)–Regulatory News:
Mainstay Medical International plc (“Mainstay” or the “Company”, Euronext Paris: MSTY.PA and Euronext Dublin: MSTY.IE), a medical device company focused on commercializing ReActiv8®, an implantable restorative neurostimulation system designed to treat an underlying cause of disabling Chronic Low Back Pain, today announces that it will participate in the 13th German Spine Congress of the Deutsche Wirbelsäulengesellschaft (DWG), taking place in Wiesbaden from December 6-8. DWG will be the first medical meeting at which pivotal clinical data from the Company’s ReActiv8-B clinical study will be discussed.
A distinguished faculty of physicians, each of whom has deep experience with Reactiv8, will present the clinical data and the scientific background of the therapy, and describe commercial treatment of patients in Germany:
- Dr. Jörg Franke, Chief, Department of Orthopedics, Klinikum Magdeburg, will chair the symposium where the results from the ReActiv8-B study will be presented, and also provide his experience with ReActiv8 in Germany;
- Dr. Chris Gilligan, Chief, Division of Pain Medicine, Department of Anaesthesiology, Perioperative and Pain Medicine Brigham & Women’s Hospital, Assistant Professor of Anaesthesia, Harvard Medical and Principal investigator of the study, will present the pivotal clinical data from the ReActiv8-B study;
- Dr. Jan Schilling, Chief of Spine and Neurosurgery, Tabea Hospital in Hamburg, will introduce the scientific background and the underlying physiological mechanisms of this new restorative treatment for chronic low back pain; and
- Dr. Ardeshir Ardeshiri, Chief of Spine Surgery at Klinikum Itzehoe, will present his “real world” experience with ReActiv8 in Germany via his initial series of patient outcomes.
“As Germany is our first commercial market for ReActiv8, I am excited to be unveiling the data from our ReActiv8-B clinical study to the scientific community at the German Spine Congress,” said Jason Hannon, Chief Executive Officer of Mainstay. “We believe the long-term clinical results demonstrated in the study are compelling, particularly that we are seeing more than 60% of the study patients reporting greater than 50% pain relief at one year. We look forward to discussing the results with the attending physicians, and we plan to leverage the study results in continuing to drive our commercial business in Germany and more broadly in Europe.”
“The data from the ReActiv8-B clinical study through one year, and the favourable safety profile demonstrate that ReActiv8 is a viable restorative treatment option for patients with chronic low back pain,” said Dr. Franke. “ReActiv8 has the potential to provide long-term pain relief to this patient population, which suffers from a lack of available treatment alternatives.”
The ReActiv8 B clinical study is an international, multi-center, prospective, randomized, active-controlled, blinded trial with one-way cross-over, conducted under an Investigational Device Exemption (IDE) from the U.S. Food & Drug Administration (FDA). A total of 204 patients were implanted with ReActiv8 at leading study centers in the U.S., Europe and Australia and randomized 1:1 to therapy or control 14 days after implant. The initial results from this clinical study were announced by Mainstay on November 19, 2018.
“The results from this study confirm the results we have seen in our own experience with chronic mechanical low back pain patients with ReActiv8,” said Dr. Schilling. “There is an urgent need for new, effective therapies to treat long-term, mechanical low back pain, and the data from the studies demonstrate that ReActiv8 can provide sustained pain reduction over time.”
Mainstay will hold a ReActiv8-B symposium at which the physician faculty will make their presentations. The symposium will take place on Friday, December 7 from 13:00 – 14:30 in Studio 1.2 A & B at the Rhein Main Congress Center. Seating is limited. Pre-registration is available through www.reactiv8-B.de.
The Company will also have a booth at the DWG Congress, location number 26, and Mainstay leadership and the symposium faculty will be available for further discussions throughout the conference.
About Mainstay
Mainstay is a medical device company focused on commercializing an innovative implantable restorative neurostimulation system, ReActiv8®, for people with disabling Chronic Low Back Pain (CLBP). The Company is headquartered in Dublin, Ireland. It has subsidiaries operating in Ireland, the United States, Australia, Germany and the Netherlands, and is listed on the regulated market of Euronext Paris (MSTY.PA) and the ESM of Euronext Dublin (MSTY.IE).
About the ReActiv8-B Study
The ReActiv8-B Study is an international, multi-center, prospective, randomized, sham-controlled, blinded trial with one-way crossover conducted under an Investigational Device Exemption (IDE). In summary, this means that eligible patients had baseline data collected and then following verification that the enrollment criteria were met, ReActiv8 was implanted. At the 14-day post implant follow up visit, half the patients were randomized to receive appropriately programmed stimulation (the treatment arm), and half were randomized to receive sham stimulation/low stimulation (the control arm). Information about the study can be found at https://clinicaltrials.gov/ct2/show/study/NCT02577354.
About Chronic Low Back Pain
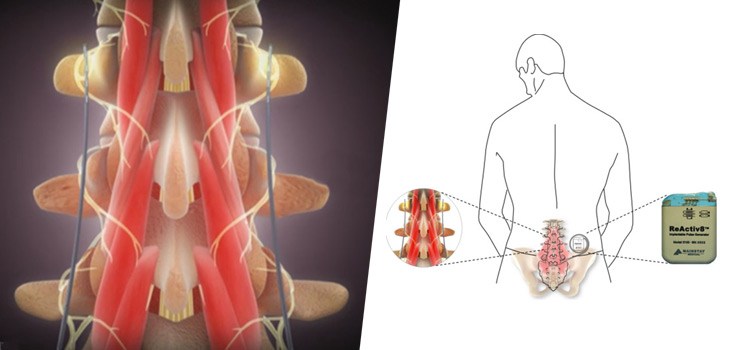
One of the recognized root causes of CLBP is impaired control by the nervous system of the muscles that dynamically stabilize the spine in the low back, and an unstable spine can lead to back pain. ReActiv8 is designed to electrically stimulate the nerves responsible for contracting these muscles and thereby help to restore muscle control and improve dynamic spine stability, allowing the body to recover from CLBP.
People with CLBP usually have a greatly reduced quality of life and score significantly higher on scales for pain, disability, depression, anxiety and sleep disorders. Their pain and disability can persist despite the best available medical treatments, and only a small percentage of cases result from an identified pathological condition or anatomical defect that may be correctable with spine surgery. Their ability to work or be productive is seriously affected by the condition, and the resulting days lost from work, disability benefits and health resource utilization put a significant burden on individuals, families, communities, industry and governments.
Further information can be found at www.mainstay-medical.com
CAUTION – in the United States, ReActiv8 is limited by federal law to investigational use only.
Forward looking statements
This announcement includes statements that are, or may be deemed to be, forward looking statements. These forward looking statements can be identified by the use of forward looking terminology, including the terms “anticipates”, “believes”, “estimates”, “expects”, “intends”, “may”, “plans”, “projects”, “should”, “will”, or “explore” or, in each case, their negative or other variations or comparable terminology, or by discussions of strategy, plans, objectives, goals, future events or intentions. These forward looking statements include all matters that are not historical facts. They appear throughout this announcement and include, but are not limited to, statements regarding the Company’s intentions, beliefs or current expectations concerning, among other things, the data from the ReActiv8-B clinical study, the Company’s plans in relation to that data, and the Company’s results of operations, financial position, prospects, financing strategies, expectations for product design and development, regulatory applications and approvals, reimbursement arrangements, costs of sales and market penetration and other commercial performance.
By their nature, forward looking statements involve risk and uncertainty because they relate to future events and circumstances. Forward looking statements are not guarantees of future performance, and the actual results of the Company’s operations, and the development of its main product, the markets and the industry in which the Company operates, may differ materially from those described in, or suggested by, the forward looking statements contained in this announcement. In addition, even if the Company’s results of operations, financial position and growth, and the development of its main product and the markets and the industry in which the Company operates, are consistent with the forward looking statements contained in this announcement, those results or developments may not be indicative of results or developments in subsequent periods. A number of factors could cause results and developments of the Company to differ materially from those expressed or implied by the forward looking statements including, without limitation, the successful launch and commercialization of ReActiv8, the outcome of the ReActiv8-B Clinical Study, the outcome of the Company’s interactions with the FDA on a PMA application for ReActiv8, general economic and business conditions, global medical device market conditions, industry trends, competition, changes in law or regulation, changes in taxation regimes, the availability and cost of capital, the time required to commence and complete clinical trials, the time and process required to obtain regulatory approvals, currency fluctuations, changes in its business strategy, and political and economic uncertainty. The forward-looking statements herein speak only at the date of this announcement.