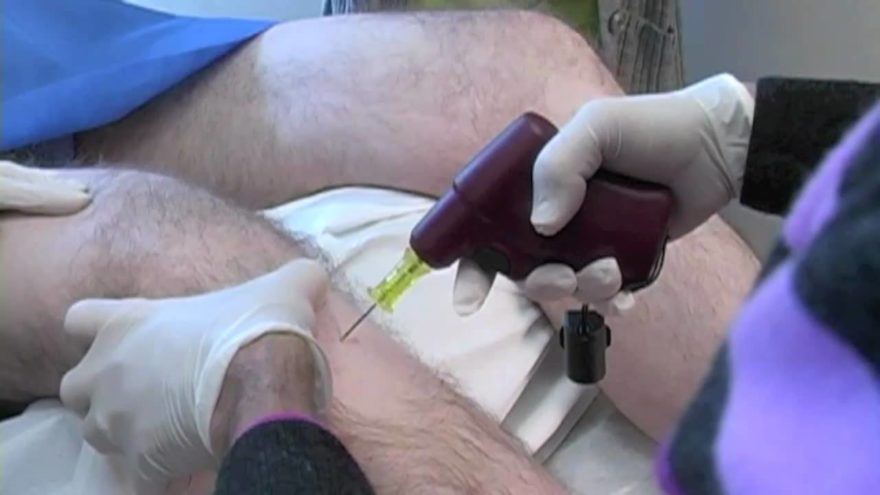
LONDON–(BUSINESS WIRE)–According to the latest market study released by Technavio, the surgical navigation systems market in the US is expected to grow at a CAGR of almost 6% during the forecast period.
This research report titled ‘Surgical Navigation Systems Market in the US 2016-2020’ provides an in-depth analysis of the market in terms of revenue and emerging market trends. This market research report also includes up to date analysis and forecasts for various market segments and all geographical regions.
One of the most important factors driving this market is the expansion of outpatient settings. Studies have proved computer-assisted surgeries to be more precise and less risky, resulting in improved quality of life for the patient. The procedures are pre-programmed into a surgical navigation system, and can be carried out in outpatient settings with the assistance of an experienced surgeon. This has resulted in an increased number of ambulatory surgical centers (ASCs) where even the most complex spinal surgeries can be performed in a safe and sterilized setting.
Other key drivers for this market are the growing popularity of minimally invasive surgeries, ever broadening applications of surgical navigation systems, and innovative marketing strategies used by vendors. Due to the increasing demand for surgical navigation systems, manufacturers are focusing on developing portable and lightweight systems equipped with the latest, cutting-edge software capabilities.
Request a sample report: http://www.technavio.com/request-a-sample?report=55087
Technavio’s sample reports are free of charge and contain multiple sections of the report including the market size and forecast, drivers, challenges, trends, and more.
Based on application, the report categorizes the surgical navigation systems market in the US into the following segments:
- Neurosurgical navigation systems
- Orthopedic surgical navigation systems
- ENT surgical navigation systems
- Spine surgical navigation systems
Neurosurgical navigation systems
“The neurosurgical navigation systems market represents the largest segment of the surgical navigation systems market in the US. This system is ideal for surgeries related to cranial tumors and neurological disorders. There has been an increase in the use of frameless stereotaxy and MRI image-guided navigation for obtaining brain biopsies and in tumor resurrection procedures. This has clearly demonstrated improved surgical outcomes for complex spinal surgeries,” says Barath Palada, one of the lead analysts at Technavio for medical devices research.
With the increase in the volume of neurosurgical procedures across the US and high adoption of minimally invasive surgeries, the demand for neurosurgical navigation systems is on the rise. These systems have also shown to lessen the extent of neurological functioning when compared to traditional surgeries. This is mainly due to the availability exact mapping for a variety of cortical functions like sensory, motoric, speech, and language functions, and for visualizing subcortical pathways for both benign and malignant brain tumors.
Orthopedic surgical navigation systems
The orthopedic surgical navigation market represents the second largest surgical navigation systems in the US. Statistics show that the number of people suffering from musculoskeletal diseases is growing. The American Academy of Orthopedic Surgeons (AAOS), hospital discharge data has shown that an increasing number of patients are undergoing knee replacements and this rate has jumped up by 120% over a 10-year period, and overall hip replacement cases have also doubled in the past decade.
In this scenario, with a growing number of orthopedic surgeries, the surgical navigation system will be ideal for several procedures including joint arthroplasties, total hip arthroplasty, total shoulder arthroplasty, limb-salvage techniques and fracture surgeries. Computer-assisted orthopedic surgery (CAOS) system comprises of 3D image-guided and non-image based navigation systems, intraoperative fluoroscopic navigation, robotic-assistance tools, and intraoperative visualization devices.
ENT surgical navigation systems
“The ENT surgical navigation market represents the newest and fastest growing segment in the surgical navigation systems market in the US. With the rising number of ASCs, the outpatient setting of the surgical navigation system is establishing itself as a popular option for most patients opting for ENT. In 2015, 92% of the ear and 87% of the nasal surgeries were performed in ASCs in an outpatient setting,” says Barath.
ENT navigation systems are especially beneficial as these surgeries include working with delicate anatomies, such as optic nerves, blood vessels, eyes, and the nasal region, which are beyond the view of the endoscope. These systems help in precise navigation of surgical instruments through complex sinus passages without causing any damage to the surrounding anatomy.
Spine surgical navigation systems
Spinal fusion surgeries are performed to relieve pain arising due to injury, degenerative disc disease, spinal curvatures or arthritis. This surgery is most commonly carried out through surgical navigation systems in the US. Additionally, extremely precise placement of implants like pedicle screws is assured by computer assisted navigation when compared to other conventional surgeries. This is especially true during treatment of spinal instability caused by degenerative disc disease, spinal stenosis, spondylolisthesis, deformity, fractures, tumors, and infection. Usage of CT-guided or 3D fluoroscopy-based navigation systems has clearly demonstrated an increase in the accuracy up to 98% and lessen the need for re-operating.
The top vendors highlighted by Technavio’s research analysts in this report are:
- B. Braun Melsungen
- Brainlab
- GE Healthcare
- Medtronic
- Stryker
Browse Related Reports:
Become a Technavio Insights member and access all three of these reports for a fraction of their original cost. As a Technavio Insights member, you will have immediate access to new reports as they’re published in addition to all 6,000+ existing reports covering segments like patient monitoring devices, medical imaging, and life science research tools. This subscription nets you thousands in savings, while staying connected to Technavio’s constant transforming research library, helping you make informed business decisions more efficiently.
About Technavio
Technavio is a leading global technology research and advisory company. The company develops over 2000 pieces of research every year, covering more than 500 technologies across 80 countries. Technavio has about 300 analysts globally who specialize in customized consulting and business research assignments across the latest leading edge technologies.
Technavio analysts employ primary as well as secondary research techniques to ascertain the size and vendor landscape in a range of markets. Analysts obtain information using a combination of bottom-up and top-down approaches, besides using in-house market modeling tools and proprietary databases. They corroborate this data with the data obtained from various market participants and stakeholders across the value chain, including vendors, service providers, distributors, re-sellers, and end-users.
If you are interested in more information, please contact our media team at media@technavio.com.