September 07, 2016
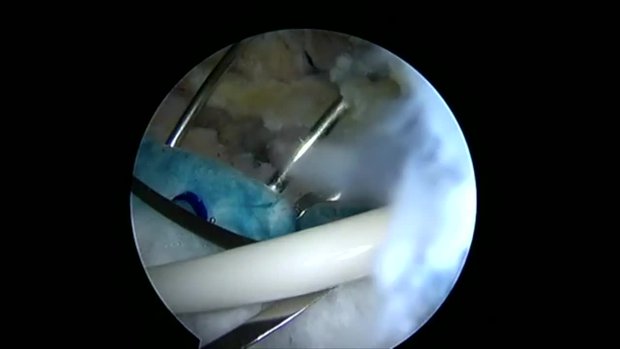
PLYMOUTH, Minn.–(BUSINESS WIRE)–Rotation Medical Inc., a medical device company focused on developing new technologies to treat rotator cuff disease, today announced that study results published in the current issue of Muscle, Ligaments and Tendons Journal showed that the company’s collagen-based bioinductive implant induced new tissue formation in all study patients with rotator cuff tears. The Rotation Medical rotator cuff system is a novel, implant-based solution for rotator cuff repair and a new alternative to traditional surgical repair.
The study assessed the ability of Rotation Medical’s bioinductive implant to induce new tissue formation and limit tear progression when placed on the bursal surface of partial thickness cuff tears. The implant induced significant new tissue formation in all 13 patients by three months (mean increase in tendon thickness 2.2 ± 0.26 mm), and the tissue matured over time and became radiologically indistinguishable from the underlying tendon. No tear progression was observed on MRI in any of the patients during the 24-month post-operative period. All patients’ Constant and American Shoulder and Elbow Society clinical scores improved significantly over time.1
“Partial-thickness rotator cuff tears frequently enlarge due to increased local strain and often progress to full-thickness tears,” said Dr. Desmond John Bokor, lead study investigator and associate professor in the Department of Orthopaedic Surgery at Macquarie University in Australia. “The results of this study demonstrate the ability of the bioinductive implant to induce new tendon-like tissue, enabling partial-thickness rotator cuff tears to decrease in size and in most cases disappear. The ability to heal partial-thickness rotator cuff defects, and thus prevent tear propagation and progressive tendon degeneration, represents a novel interventional treatment paradigm for these lesions.”
Rotator cuff damage is the most common source of shoulder pain, affecting more than 4 million people annually in the U.S. Traditional approaches to treating degenerate or torn rotator cuffs often do not address the poor quality of the underlying tendon tissue, and a significant number of these tendons, after standard treatment, either degenerate further and/or re-tear. Cleared by the U.S. Food and Drug Administration in March 2014, the Rotation Medical bioinductive implant is designed to address this limitation by inducing new tissue growth at the site of implantation, resulting in increased tendon thickness and healing of tendon defects with new tissue growth. The collagen-based implant is about the size of a postage stamp and it is part of the Rotation Medical rotator cuff system, which also includes disposable instruments that allow the arthroscopic procedure to be performed easily and quickly.
“This study is further evidence that the Rotation Medical rotator cuff system has the potential to transform the treatment of rotator cuff disease,” said Martha Shadan, president and CEO of Rotation Medical. “Our bioinductive implant addresses both the biomechanics and biology required to heal a rotator cuff tendon tear, preventing rotator cuff tears from becoming larger over time, reducing the incidence of re-tears and, in some cases, shortening patient recovery time.”
The study, “Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: a 2-year MRI follow-up,” adds to the growing body of literature supporting the use of the bioinductive implant as a novel treatment for rotator cuff partial thickness tears. Additional publications and information about the Rotation Medical rotator cuff system are available on the company’s website.
About the Study
The implant was inserted via arthroscopic surgery. A total of 13 patients with intermediate (3-6 mm) to high-grade (>6 mm) partial thickness cuff tears completed two years of follow-up. At three, six, 12, and 24 months postoperatively, tendon thickness, defect size and quality were evaluated using magnetic resonance imaging (MRI), and clinical outcomes were assessed using the Constant and American Shoulder and Elbow Society scores. The partial-thickness cuff tears showed consistent filling of the defects, with complete healing in seven patients at 12 months, and a progressive improvement in tendon quality in the remaining patients. No tear progression was observed by MRI in any of the patients at 24 months. All clinical scores improved significantly over time. At 24 months, 12 of 13 patients (92 percent) had satisfactory or better results.
About Rotation Medical
Rotation Medical Inc. was founded in 2009 and is committed to improving the treatment of rotator cuff disease with the Rotation Medical rotator cuff system, a breakthrough technology that has the potential to prevent rotator cuff disease progression and reduce re-tears by inducing the growth of new tendinous tissue. The company is privately held and funded by New Enterprise Associates (NEA), Life Sciences Partners (LSP) and Pappas Ventures. For more information, visit www.rotationmedical.com.
________________________
1 Bokor DJ, Sonnabend D, Deady L, Cass B, Young A, Van Kampen C, Arnoczky S. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: a 2-year MRI follow-up. Muscle Ligaments Tendons J. 2016 May 19;6(1):16-25. doi: 10.11138/mltj/2016.6.1.016.
Contacts
Merryman Communications
Joni Ramirez, 323-532-0746
joni@merrymancommunications.com