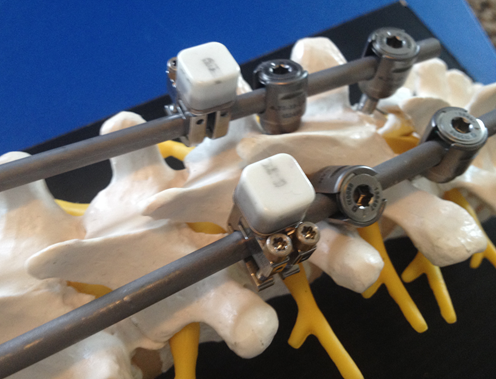
AKRON, Ohio, June 28, 2017 (GLOBE NEWSWIRE) — Intellirod Spine, the spinal implant company developing disposable and implantable wireless RFID sensor technology for monitoring spinal rod strains, secured additional equity financing from new and existing investors led by the OhioHealth Innovation Development Fund and Queen City Angel First Fund V. Funds will be used to reach key milestones toward the commercialization of the company’s sensor technologies and related lumbar fusion implants.
Intellirod Spine recently had the first human use of LOADPRO™ in an approved clinical study at Norton Leatherman Spine Center by Dr. Jeffrey Gum. The company’s intraoperative LOADPRO™ sensor was used to record strains on spinal rods during a kyphotic deformity correction surgery. Dr. Gum says, “We are very excited about the start of the clinical trial. Although the data was blinded to us intraoperatively, the post-hoc review shows promising results. This exciting technology will provide valuable information that will help guide intraoperative decision making and potentially lead to non-invasive or non-ionizing information to help assess construct stability and fusion status. We look forward to the progress of the study”. This is meant to help surgeons quantify their tactile feel of the forces being applied during the deformity correction. The company is pursuing a de novo 510(k) at the FDA and is simultaneously seeking a CE Mark for LOADPRO™. According to Ric Navarro, Intellirod CEO, “This new information will not only help the surgeon but will help us with our FDA and CE Mark approvals”. Two additional clinical sites include Grant Medical Center in Columbus, Ohio and the Cleveland Clinic in Cleveland, Ohio. The company is also actively seeking a fourth site.
The company is announcing its entrance into the Digital Health space with new intellectual property paving the way toward in-home remote monitoring through its IntelliBrace™ project. They anticipate being the first to offer autonomous data collection from their wireless implant, the ACCUVISTA™ rod strain sensor. This will position the company to provide caregivers more information about the state of the implant and other measured parameters aimed at improving patient compliance and decreasing mobility related complications. Dr. Chris Karas, a neurosurgeon at OhioHealth and inventor of the IntelliBrace™ concept, says, “Intellirod is focused on collecting the data surgeons need to achieve better outcomes for our patients. This type of technology is currently lacking, but is absolutely necessary to get people back to work and back to life”.
The OhioHealth Innovation Development fund, which contributed to this project, supports physicians, nurses and associates working to bring their product ideas to life, while also giving them the opportunity to retain ownership of their intellectual property. “Dr. Karas, as an inventor of the IntelliBrace™, is a perfect example of how this OhioHealth fund can work. We are hoping this success story will encourage other brilliant, inventive minds at OhioHealth to dream big, and see those dreams turn into reality with funding help,” said Alan Nelson, OhioHealth Treasury Vice President.
Mr. Navarro stated the company is excited to make this evolution with its platform technology into the lower cost of care environment of the home to aid in the patient’s rehabilitation. “Not only does IntelliBrace™ add new functional benefits but it will create cloud database opportunities for aggregating and interpreting data toward new norms in remote spine patient care”, says Mr. Navarro.
Intellirod has also signed a license agreement with the Cleveland Clinic to take its microelectronics and sensing know-how and technology into other parts of the spine with a therapeutic device with an integrated sensor. The company is leveraging its platform of inductive powering and wireless communication into a therapeutic device to create a next generation “Smart” implant. Cleveland Clinic’s Dr. Michael Steinmetz, who is a paid member of Intellirod’s scientific advisory board, says, “The LOADPRO System will provide data for enhancing surgical techniques and reducing complications and costs. By sensing the progress of fusion through a sensor-enabled device we commonly use in spine fusions, our goal is to improve post-operative care.”
About Intellirod Spine
Intellirod Spine™ (formerly OrthoData Inc.) based in Akron, Ohio was founded by renowned spine surgeon Rolando M. Puno, M.D. and professors from the University of Louisville. The company is developing a wireless microelectronic spinal rod strain sensor and inductive power platform. This innovative strain monitoring system will allow spine surgeons to objectively assess the strain on spinal rods intraoperatively and postoperatively. www.intellirodspine.com
About Queen City Angels
The Queen City Angels (QCA) is a group of more than 50 experienced accredited investors who provide funding, support and guidance to early-stage growth companies in the Cincinnati area and surrounding region. QCA members, which include former C-level executives and entrepreneurs, draw from their personal operating and management experience to evaluate opportunities and provide on-going mentoring to young businesses with exceptional growth potential. Since 2000, QCA members have directly invested approximately $50 million in nearly 80 portfolio companies. The total capital invested in these companies, including QCA members’ capital, syndication partners’ capital, follow-on venture capital funds and venture debt is in excess of $410,000,000. CB Insight recently ranked QCA second out of 370 national angel organizations. For additional information, visit www.qca.com
About OhioHealth
OhioHealth is a nationally recognized, not-for-profit, charitable, healthcare outreach of the United Methodist Church.
Based in Columbus, Ohio, OhioHealth has been recognized as one of the top five large health systems in America by Truven Health Analytics, an honor it has received six times. It is also recognized by FORTUNE Magazine as one of the “100 Best Companies to Work For” and has been for 10 years in a row, 2007-2016.
Serving its communities since 1891, it is a family of 28,000 associates, physicians and volunteers, and a network of 11 hospitals, 50+ ambulatory sites, hospice, home-health, medical equipment and other health services spanning a 47-county area.
OhioHealth hospitals include OhioHealth Riverside Methodist Hospital, OhioHealth Grant Medical Center, OhioHealth Doctors Hospital, OhioHealth Grady Memorial Hospital, OhioHealth Dublin Methodist Hospital, OhioHealth Hardin Memorial Hospital, OhioHealth Marion General Hospital, OhioHealth O’Bleness Hospital, OhioHealth Mansfield Hospital, OhioHealth Shelby Hospital and OhioHealth Rehabilitation Hospital.
For more information, please visit our website at www.ohiohealth.com.
Contact Information:
Jennifer Dietrich, Intellirod Spine
phone: 234-678-8965 / email: jdietrich@intellirodspine.com
www.intellirodspine.com