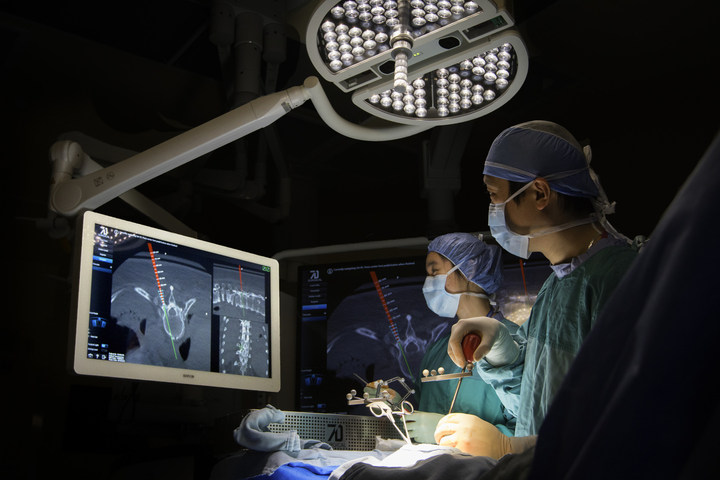
TORONTO, Jan. 23, 2017 /PRNewswire/ — 7D Surgical announced today that it has received both 510(k) clearance from the U.S. Food and Drug Administration (FDA) and a medical device license from Health Canada enabling the North American commercial launch of its innovative Machine-vision Image Guided Surgery (MIGS™) system for spine surgery, the 7D Surgical System.
The 7D Surgical System employs cutting-edge 3D optical technologies and machine vision algorithms to eliminate the long-standing barriers to adoption of existing surgical navigational platforms. This new technology can easily register spinal surgery patients automatically using only visible light. Unlike time-consuming conventional image guided surgery (IGS) systems that depend on intraoperative radiation, this new platform can achieve an incredibly fast surgical workflow for spine procedures.
“When navigating the spine, surgeons traditionally have had two time-consuming and expensive IGS options: systems that rely on intraoperative radiation emitting devices or systems that utilize laborious manual point matching techniques,” said Beau Standish, Chief Executive Officer of 7D Surgical. “We believe the inefficiencies of these systems have limited the adoption of IGS in spine procedures to less than 20%. 7D Surgical’s MIGS™ system has now removed these barriers, providing surgeons and their hospitals with a superior product option.”
The 7D Surgical System enables near-instantaneous Flash Registration™ of the patient’s anatomy. “Guided by our product philosophy of ‘surgeons designing for surgeons’, we have achieved an unprecedented entire workflow time of less than 20 seconds for de novo spinal registration, unheard of in the spinal IGS world where such registration can interrupt surgery for up to 30 minutes,” said Dr. Victor Yang, President and Chief Scientific Officer of 7D Surgical, Senior Scientist at Sunnybrook Research Institute, and Staff Neurosurgeon at Sunnybrook Health Sciences Centre in Toronto, where prototype MIGS™ technology has been used in more than 160 patients in clinical trials.
The MIGS™ navigation technology is embedded in an onboard overhead surgical light, which eliminates line of sight frustrations in the operating room, and is manifested in simplified yet powerful software, which is controlled by the surgeon using only a foot pedal. “Our surgeons don’t rely on non-sterile personnel to operate the technology. Our surgeons are in total control,” said Yang.
“Image guided surgery technology has finally caught up to the needs of a practicing spine surgeon,” said Dr. Frank Cammisa, Chief Emeritus of the Spine Service at Hospital for Special Surgery in New York City. “7D Surgical’s new MIGS™ system appears to provide a faster, radiation-free alternative to existing options. It could be an important new tool in expanding the use of IGS in spine procedures.” He added, “The 7D Surgical System reduces the overall cost and footprint required to navigate the spine. It’s a win-win for the surgeon and the hospital.”
With both U.S. and Canadian regulatory authorization, 7D Surgical has commenced execution of its North American commercialization strategy. “We are delighted to have achieved these regulatory milestones in line with our expectations and planning,” said Standish. “We are confident that in demonstrating the speed and efficiency of our MIGS™ system, we will convince more surgeons to employ IGS in their spine procedures.”
About 7D Surgical
7D Surgical is a privately-owned Toronto based company that develops advanced optical technologies and machine vision-based registration algorithms to improve surgical workflow and patient care. 7D Surgical’s flagship FDA 510(k)-cleared and Health Canada approved MIGS™ system delivers profound improvement to surgical workflows in spine surgery, providing the promise of similar future advancements in other surgical specialties.
Contact:
Beau Standish, CEO
7D Surgical
+1 647 484-0078
www.7Dsurgical.com
140332@email4pr.com
Forward-looking Statements
This press release contains forward-looking statements regarding, among other things, statements pertaining to expectations, goals, plans, objectives, and future events. 7D Surgical intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934, and the Private Securities Reform Act of 1995. In some cases, forward-looking statements can be identified by the following words: “may,” “can,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “promise,” “continue,” “ongoing,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements are based on the current estimates and assumptions of our management as of the date of this press release and are subject to risks, uncertainties, changes in circumstances, assumptions, and other factors that may cause actual results to differ materially from those indicated by forward-looking statements, many of which are beyond 7D Surgical’s ability to control or predict. Given these uncertainties, undue reliance should not be placed on these forward-looking statements. 7D Surgical does not undertake any obligation to release publicly any updates or revisions to these forward-looking statements to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events.
“MIGS™”, “Flash Registration™,” as well as the “7D” logo, whether standing alone or in connection with the words “7D Surgical” are protected trademarks of 7D Surgical.
SOURCE 7D Surgical
Related Links