NORCROSS, Ga., July 5, 2018 /PRNewswire/ — Lipogems, a leader in medical technologies for orthopaedic physicians, announced U.S. Food and Drug Administration (FDA) clearance of the company’s Lipogems Microfragmented Adipose Tissue Transplant System. Federal regulators have cleared the way for this exciting new device and technology to be used in orthopaedic and arthroscopic procedures. Lipogems successfully uses a patient’s own body fat (known clinically as adipose tissue) to assist in the healing process and, in some cases, used in conjunction with traditional orthopaedic surgery. Lipogems is attractive to orthopaedic physicians because it is compliant with the latest FDA guidelines and is cleared for use in orthopaedics and arthroscopic surgery unlike many other technologies.
“The use of reparative technologies in orthopaedics is rapidly expanding and medicine is forced to keep up with patient demands and the forces that are placed on their bodies. This is true for all types of patients from young athletes to professional athletes, from weekend warriors to the elderly,” said world renowned sports medicine surgeon Dr. Champ Baker, Jr., of the respected Hughston Clinic in Columbus, GA. “As a result, we are understanding more about reparative medicine options and the science behind them.
“Orthopaedic conditions are the number one cause of disability in patients in the United States and they take a toll on their physical and emotional health, and the ability to support their families. Patients are living longer and are more highly active. They are really trying to maintain their quality of life and function freely,” said Dr. Baker.
The Power of Fat
“Many patients are looking for another option to major invasive surgery. Fat has many important cells and is easy to get from the patient’s body. I believe cells from their own body are the best cells that a patient can use,” said Dr. Baker. “Lipogems may be a great option for patients who have tried physical therapy, nonsteroidal anti-inflammatory drugs, or steroid injections, and other treatments that have not provided enough relief.
“If surgery is needed, Lipogems may be ideal to help provide cushion and support to facilitate the healing environment and optimize recovery,” he added.
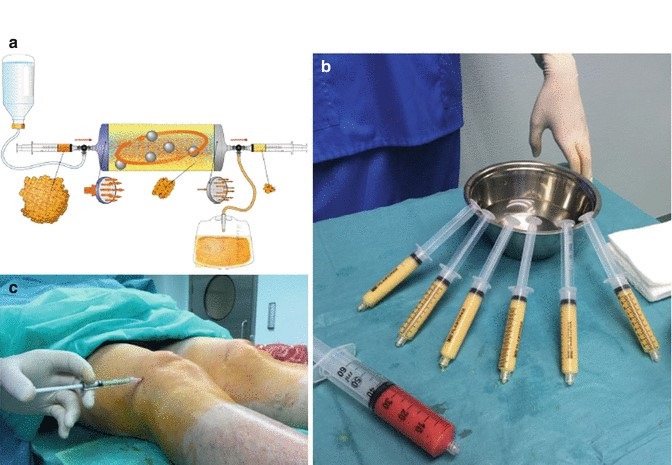
Orthopaedic physicians are always looking for convenient technologies that are compliant with current standards. The Lipogems system arrives in a comprehensive kit and uses a minimally invasive procedure to harvest, concentrate, then transfer the patient’s own adipose tissue, typically from the patient’s belly or “love handles.”
The procedure can be performed in the office or surgical setting in under an hour. The Lipogems system uses only saline to rinse and remove contaminants, typically oil, blood and cell debris, from the harvested fat. Lipogems then resizes, or “microfragments”, the tissue to an optimum size while retaining the fat tissue’s natural and beneficial properties.
The “clean” Lipogems tissue provides cushion and support for the area. In accordance with homologous use, the tissue processed in the Lipogems system may be used to facilitate the natural healing process by supporting the repair, replacement or reconstruction of damaged or injured tissue.
If a patient suffers from multiple orthopaedic conditions, additional fat can be removed (in the same procedure) and processed through the Lipogems technology to help address those additional conditions.
“For procedures performed in the office, there is minimal recovery time and the patients can leave the facility right away and go home with minimal pain medication,” Dr. Baker said. “For patients needing arthroscopic surgery, the introduction of microfragmented fat to their shoulder, hip, or knee may provide cushion and support of the damaged tissue, and optimize the surgical recovery.”
In November 2017, the FDA finalized its rules guiding the use of Human Cellular and Tissue Products. The Agency reaffirmed that the Lipogems system meets the new guidelines’ criteria for minimal manipulation of the tissue, and that it is intended for homologous use. “Fat has been used for many years in support of the repair or replacement of damaged or injured tissue,” according to Dr. Arnold Caplan of Case Western Reserve University in Cleveland, Ohio. “Fat has a high concentration of reparative cells and is a very powerful tissue. How the fat is processed makes a huge difference on the quality of the tissue and if it meets the new FDA guidelines.”
Dr. Diego Correa, Assistant Professor, Department of Orthopaedics (Division of Sports Medicine) & Diabetes Research Institute- Cell Transplant Center at the University of Miami’s Miller School of Medicine is also impressed by the Lipogems technology. “Fat has a lot of important structural and reparative cells (including pericytes, adipocytes, and other cells) and they need to be kept together to meet the new guidelines by the FDA. Lipogems is unique because it keeps the cells and tissue intact, and thus functions much like the way it does naturally in the human body—and meets the new FDA guidelines for minimal manipulation.”
Accept No Substitutes
According to Carl Llewellyn, President of Lipogems, USA, “There are many stem cell treatment centers and clinics using technologies that are not FDA cleared or approved. Many of these clinics are selling stem cell treatments unproven for safety and efficacy, and these lack FDA review and oversight.”
“In fact, many of these clinics are using a process to create a ‘stromal vascular fraction’ of cells isolated from fat tissue, which the FDA considers to be more than minimally manipulated, and thus, requiring significant regulatory oversight as an experimental drug. Lipogems is offered by leading orthopaedic physicians from around the world and encourages patients to seek care from board certified orthopaedic physicians and to ensure the technology they are using is cleared or approved by the FDA.”
Lipogems is pleased to announce its participation at the annual meeting of the American Orthopaedic Society for Sports Medicine (AOSSM), July 5-8, 2018, this year at the Manchester Grand Hyatt in San Diego. (Lipogems will occupy Booth 402 at this year’s gathering.) The AOSSM meeting draws sports medicine surgeons from around the world and spotlights scientific and clinical presentations about the latest advancements in operative and non-operative care for sports medicine patients.
Arthroscopic Surgery
Arthroscopic surgery is another treatment getting a potential boost from Lipogems. Arthroscopy is the minimally invasive surgical procedure in which an orthopaedic surgeon uses a specialized tool with a tiny camera (i.e. arthroscope) to gain access to the damaged joint. Millions of such procedures are performed each year on the hips, knees, and shoulders of patients young and old. Advancements in technology have improved arthroscopic surgical techniques and patient care. But surgeons continue to be hampered by the limited healing capacity of damaged tissue. But with Lipogems, a patient’s own microfragmented fat may be used to help provide some badly needed cushion and support to facilitate healing.
About Lipogems
Lipogems’ vision is to bring responsible, effective medical technologies to physicians and patients—and to establish the Lipogems system as a mainstream solution. Lipogems strives to provide physicians and patients with adipose tissue solutions to help maintain or restore patient lifestyles and to improve quality of life and recovery times.
These solutions may offer another option to those who may not want, or who are not candidates for, major, invasive surgery.
The Lipogems system is the company’s first product to receive 510(k) clearance in the United States and is indicated for the harvest, concentration, and transfer of autologous adipose tissue. The system meets new FDA guidelines for minimal manipulation and is intended for homologous use. The company is based in Milan, Italy and Norcross, Georgia, USA. Lipogems is distributed in 27 countries around the world.
For more information, please visit www.lipogems.eu and follow us on Facebook at Lipogems International.
SOURCE Lipogems
Related Links
http://www.understandlipogems.com