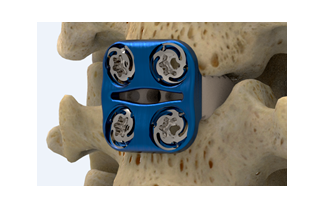
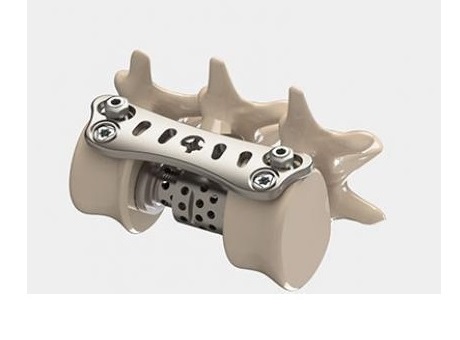
SAN DIEGO, Sept. 5, 2018 /PRNewswire/ — NuVasive, Inc. (NASDAQ : NUVA ), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally-integrated solutions, today announced the launch of its TLX® 20 degree expandable spinal interbody implant with a first-of-its-kind oblique profile designed for transforaminal lumbar interbody fusion (TLIF) procedures. This product launch is another step forward in the Company’s strategy to build a comprehensive expandable portfolio to best support optimal clinical outcomes.
The TLX 20 expandable implant’s integrated auto-lock feature allows surgeons to incrementally customize expansion up to 20 degrees of oblique lordosis and tailor implant expansion based on a patient’s clinical need, compared to many expandables in the market which only offer up to 15 degrees of lordosis. The implant profile is designed to optimally contour to a patient’s interdiscal space to help maintain coronal alignment while achieving sagittal correction, an important aspect of desired patient outcomes that has been clinically validated through research and data from NuVasive’s Integrated Global Alignment® (iGA®) platform.
In addition, the TLX 20 degree implant system includes a single, low-profile instrument to position, expand and post-pack the implant with bone graft, improving visualization into the disc space and surgical workflow. The implant also features increased tapering at the distal tip to aid insertion into a collapsed disc space, common among patients with degenerative disc disease, while minimizing the disruption to the surrounding anatomy.
“The TLX 20 system is an innovative device that can improve surgical workflow and patient outcomes by easily and actively enhancing the segmental alignment and interspace height,” said Dr. Christopher Shaffrey, neurosurgeon at University of Virginia Health System. “The compact integrated insertion device makes graduated and precise expansion easy and subsequent graft packing uncomplicated and straightforward in manner. In both open and minimally invasive surgery applications, the TLX 20 facilitates treatment of the entire range of degenerative and deformity conditions requiring interbody instrumentation.”
“The innovative, oblique profile of the TLX 20 degree expandable implant is a true differentiator in the expandable interbody market,” said Matt Link, executive vice president, strategy, technology and corporate development of NuVasive. “With this latest addition, NuVasive continues to offer our surgeon partner’s the most innovative procedurally-integrated portfolio in spine to further their treatment options for patients of varying sizes and clinical needs.”
The NuVasive expandable interbody portfolio now includes three lordotic expandable systems: TLX 15 degree and TLX 20 degree; MLX®, the medial lateral expandable interbody system, which provides an ALIF-sized footprint from a posterior approach; and XLXTM ACR®, the XLIF® lordotic expandable for anterior column realignment, which recently received 510(k) clearance. All are commercially available in the U.S.
About NuVasive
NuVasive, Inc. (NASDAQ : NUVA ) is the leader in spine technology innovation, focused on transforming spine surgery and beyond with minimally disruptive, procedurally-integrated solutions designed to deliver reproducible and clinically-proven surgical outcomes. The Company’s portfolio includes access instruments, implantable hardware, biologics, software systems for surgical planning, navigation and imaging solutions, magnetically adjustable implant systems for spine and orthopedics, and intraoperative monitoring service offerings. With over $1 billion in revenues, NuVasive has an approximate 2,400 person workforce in more than 40 countries serving surgeons, hospitals and patients. For more information, please visit www.nuvasive.com.
Forward-Looking Statements
NuVasive cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that involve risks, uncertainties, assumptions and other factors which, if they do not materialize or prove correct, could cause NuVasive’s results to differ materially from historical results or those expressed or implied by such forward-looking statements. The potential risks and uncertainties which contribute to the uncertain nature of these statements include, among others, risks associated with acceptance of the Company’s surgical products and procedures by spine surgeons, development and acceptance of new products or product enhancements, clinical and statistical verification of the benefits achieved via the use of NuVasive’s products (including the iGA platform), the Company’s ability to effectually manage inventory as it continues to release new products, its ability to recruit and retain management and key personnel, and the other risks and uncertainties described in NuVasive’s news releases and periodic filings with the Securities and Exchange Commission. NuVasive’s public filings with the Securities and Exchange Commission are available at www.sec.gov. NuVasive assumes no obligation to update any forward-looking statement to reflect events or circumstances arising after the date on which it was made.
SOURCE NuVasive, Inc.